Method for preparing hydrogen silane by using calcium hydride to conduct reduction on chlorosilane
A technology of chlorosilane and calcium hydride, which is applied in the field of chlorosilane reduction, can solve the problems of high reaction system temperature, narrow application range, and inability to obtain the target product, and achieve the effect of increasing reaction speed and yield, and rapid reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The 100mL three-neck round bottom flask was dried, connected with a mechanical stirrer, spherical condenser, constant pressure funnel and gas guiding device, then evacuated for three times to replace nitrogen, and at the same time baked with a heat gun to remove attached water vapor. Under nitrogen atmosphere, add 9.3g (0.22mol) CaH 2 , 0.38g (0.01mol) lithium aluminum hydride, 25mL diethylene glycol dimethyl ether solvent, 25g ZrO 2 Ceramic ball (φ=0.90mm). Add 25.8g (0.2mol) of Me to the constant pressure funnel 2 SiCl 2 , slowly dropwise into the reaction flask under mechanical stirring. After the dropwise addition was completed, it was heated to 50° C. and reacted for 10 h. The main reduction product obtained is Me 2 SiH 2 , The reaction yield is 97%.
Embodiment 2
[0067] The 250mL three-neck round bottom flask was dried, connected with a spherical condenser, constant pressure funnel and gas guiding device, then evacuated three times to replace nitrogen, and at the same time baked with a hot air gun to remove attached water vapor. Under nitrogen atmosphere, add 9.3g (0.22mol) CaH 2 , 0.38g (0.01mol) lithium aluminum hydride, 35mL diethylene glycol dimethyl ether solvent. Add 23.0g (0.2mol) MeSiHCl to the constant pressure funnel 2 , and slowly dropwise added to the reaction flask under magnetic stirring. After the dropwise addition was completed, heat to 50°C for 7 hours, and the product 9.1g MeSiH was collected in the liquid nitrogen cold trap 3 , the yield is close to 100%.
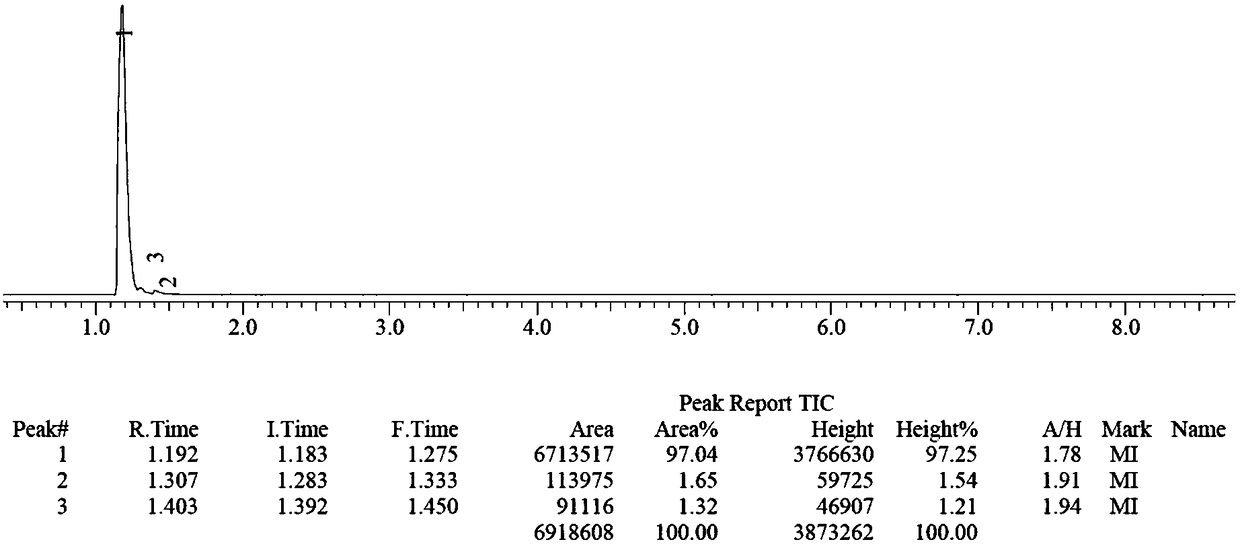
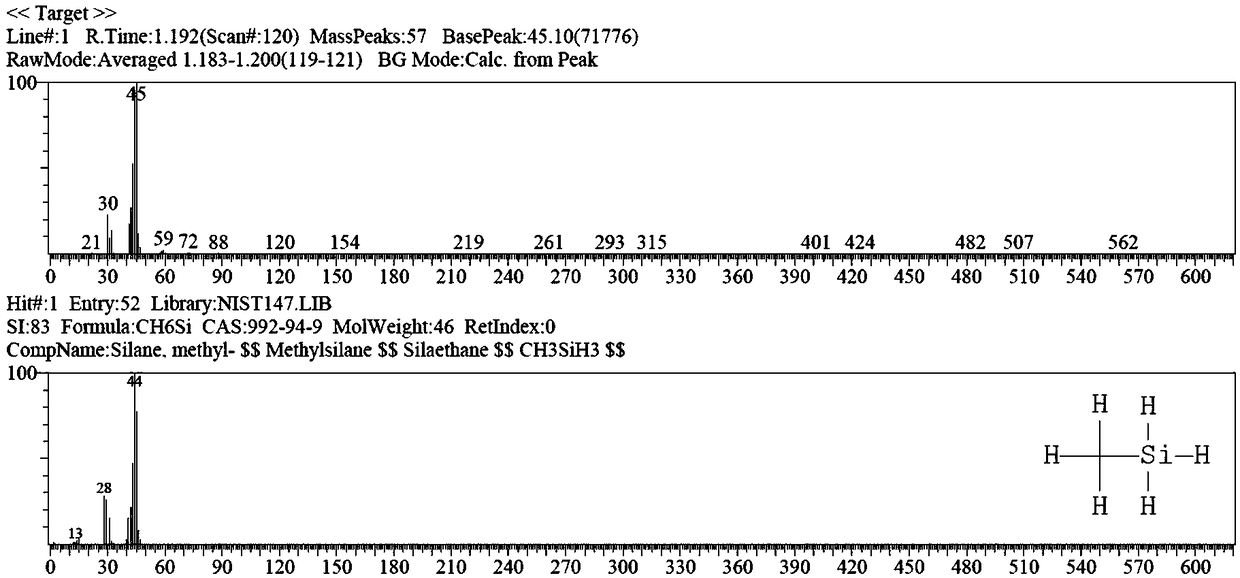
[0068] figure 1 is the chromatogram of the product, figure 2 for figure 1 The mass spectrum of the middle peak 1 confirms that the main peak 1 is methylsilane, according to figure 1 It can be calculated that the purity of methylsilane in the product reache...
Embodiment 3
[0070] The 250mL three-neck round bottom flask was dried, connected with a spherical condenser, constant pressure funnel and gas guiding device, then evacuated three times to replace nitrogen, and at the same time baked with a hot air gun to remove attached water vapor. Under nitrogen atmosphere, add 9.3g (0.22mol) CaH 2 , 0.38g (0.01mol) lithium aluminum hydride, 35mL butyl ether solvent. Add 23.0g (0.2mol) MeSiHCl to the constant pressure funnel 2 , and slowly dropwise added to the reaction flask under magnetic stirring. After the dropwise addition is completed, heat to 50°C for 7h to obtain the product MeSiH 3 , the product yield reaches 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com