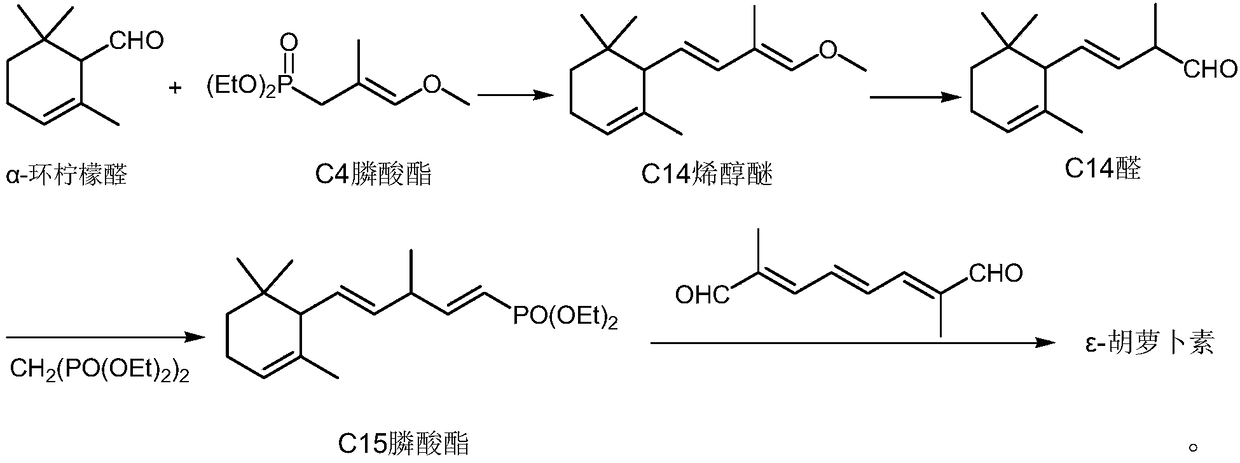
New synthesis method of epsilon-carotene
A technology of carotene and synthesis method, which is applied in the new synthesis field of ε-carotene, can solve the problems of difficulty in the source of raw material C4 phosphonate, influence on product quality of carotene, influence on large-scale application, etc., and achieves good industrialization value and cost. Low, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
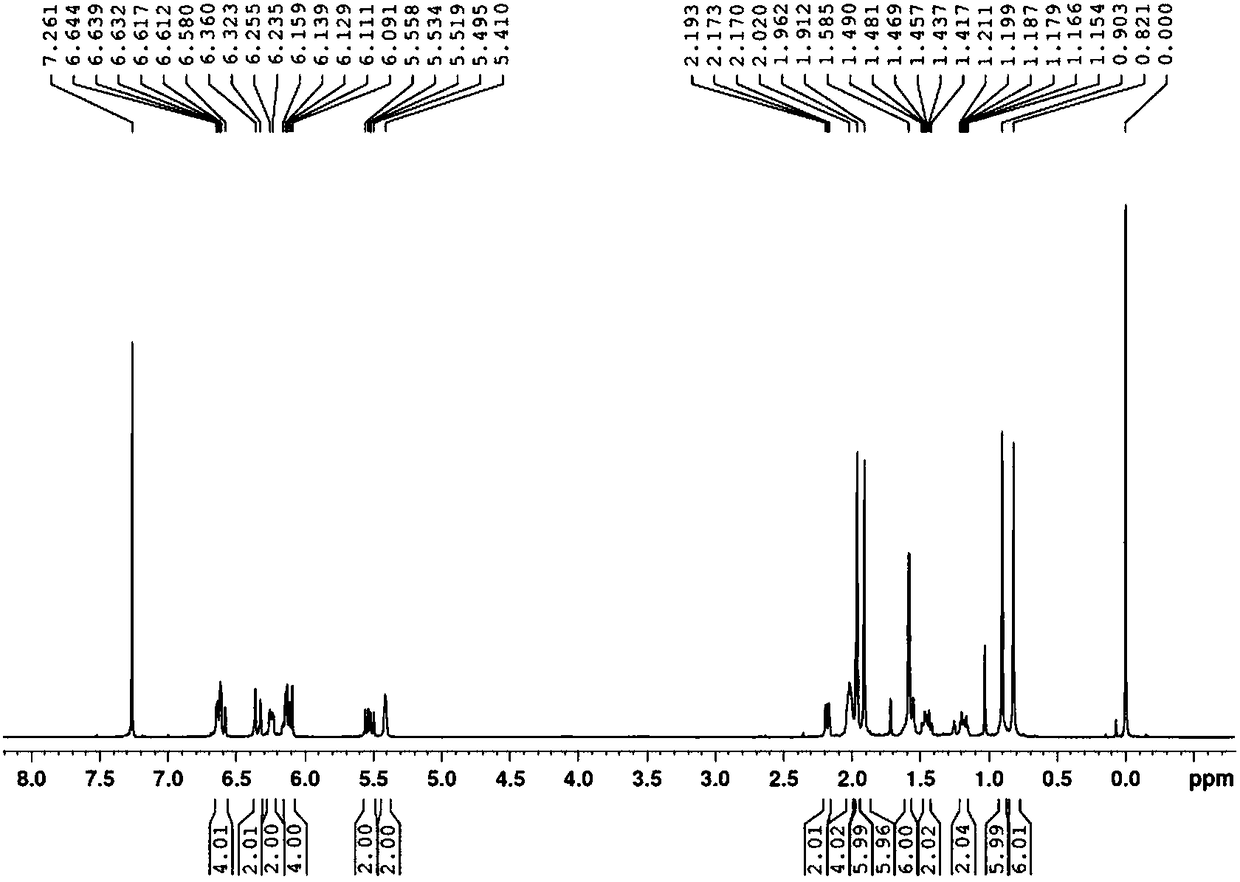
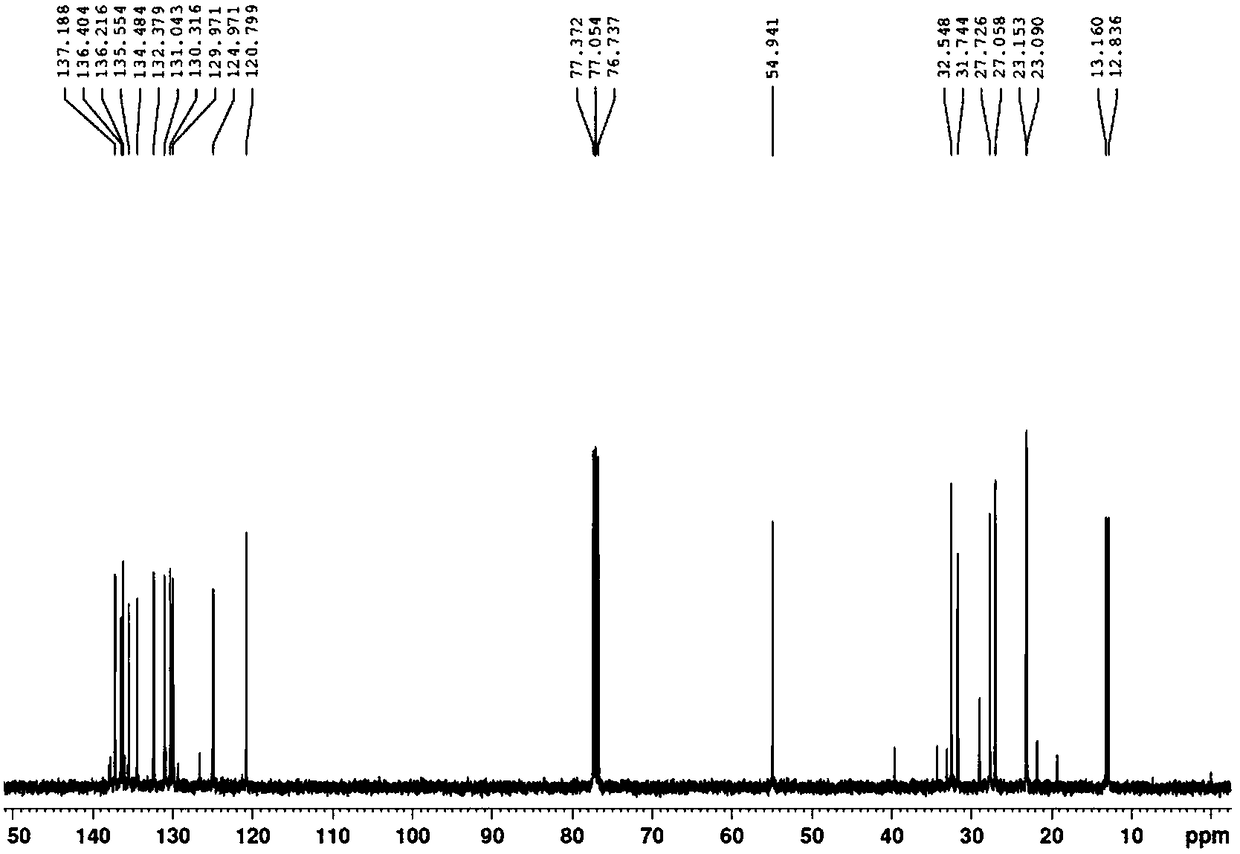
[0030] Under nitrogen protection, in a 500mL three-necked flask, add potassium tert-butoxide (32.5g, 0.288mol) and a mixture of 100mL tetrahydrofuran and dimethyl sulfoxide (the volume ratio of the two is 8:1), start the mechanical stirring, wait until the temperature When cooling to -30°C, add the decacarbon bisphosphonate (53.1g, 0.13mol) dropwise, keep at -30-25°C, drop it for about half an hour, continue to heat and stir for about 1 hour to make the decacarbon bisphosphonate Complete dissociation into the corresponding carbanion. Then, at -30-25°C, pentadecaldehyde (56.7 g, 0.26 mol) was added dropwise, and the dropwise addition was completed in about 1 hour. The stirring was continued for about half an hour, followed by gas chromatography until the reaction was completed. Add 80 mL of water and 150 mL of diethyl ether, stir for 10 minutes, separate layers, wash the diethyl ether layer with 5% aqueous sodium chloride solution (3*50 mL), dry the organic layer with anhydrous...
Embodiment 2- Embodiment 8
[0034] Similar to Example 1, the difference is only to use different lye, solvent and temperature, specifically as shown in the following table:
[0035]
[0036]
[0037] In summary, the present invention has the following advantages:
[0038] 1. The preparation method provided by the present invention has the characteristics of simple process route, convenient operation, high yield, low cost, and recyclable by-products, etc., and has good industrial value.
[0039] 2. The present invention adopts the addition of deionized water to remove the by-product diethyl phosphonate in a layered manner to achieve the effect of improving the purity, and at the same time helps to recycle the by-product waste water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com