Synthetic method of tauroursodeoxycholic acid
A technology of tauroursodeoxycholic acid and tauroursodeoxycholic acid, which is applied in the field of synthesis of tauroursodeoxycholic acid, can solve the problems of complex substances, large pollution, high production cost, etc., and achieve good application Prospect, easy industrialization, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
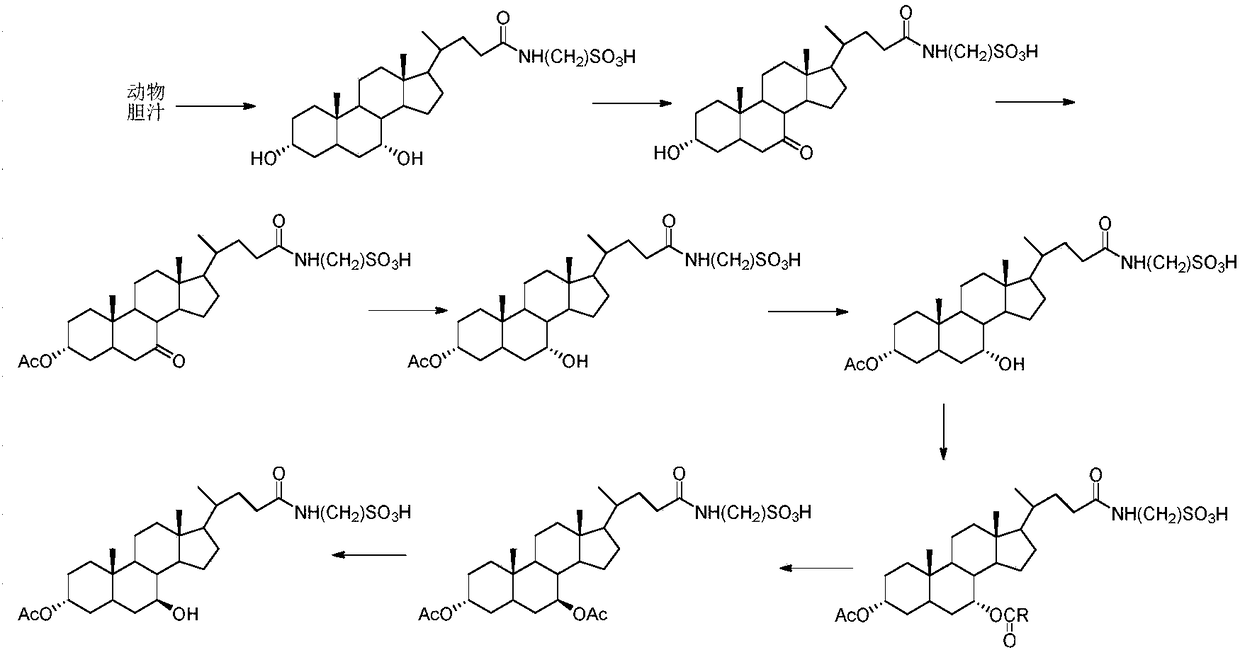
[0025] (1) Take 100g of chicken gall paste, add 500mL of water and stir to dissolve, centrifuge, filter, wash the filtrate three times with ethyl acetate (200mL*3), collect the lower layer solution; add 2-3 times of ethanol to the filtrate, centrifuge, filter, and filtrate Concentrate to dryness, add 200ml ethanol, centrifuge, filter, adjust the pH of the filtrate to 2-5 with hydrochloric acid, evaporate to dryness, add pure water to dissolve the concentrate completely, and collect taurine goose with a purity of more than 90% The fraction of deoxycholic acid was concentrated to obtain 10.3 g of the target compound with a purity greater than 90%.
[0026] (2) Dissolve taurochenodeoxycholic acid (10.0 g, 18.4 mmol) in 50 mL of acetone aqueous solution (acetone (V) / water (V) = 4 / 1), and slowly add N in batches after the dissolution is complete. -Bromosuccinimide (5.6g, 30mmol), stirred at room temperature for 4h; sampled on TLC plate, and the reaction was completed after the disa...
Embodiment 2
[0033] (1) The steps are the same as in Example 1 (1).
[0034] (2) Dissolve taurochenodeoxycholic acid (10.0g, 18.4mmol) in 50mL of acetone aqueous solution (acetone (V) / water (V) = 4 / 1), and slowly add hydrogen peroxide in batches after the dissolution is complete (3ml, 30mmol), stirred at room temperature for 4h; sampling TLC plate, until the raw material point disappears to end the reaction, distill off acetone under reduced pressure, extract with ethyl acetate, separate the organic layer, concentrate the aqueous phase to dryness to obtain the crude product, further water / Acetone (1:10 (v:v)) mixed solvent was recrystallized to obtain 7.3 g of 3α-hydroxy-7-carbonyl-cholanoyl-N-taurine.
[0035] (3) The steps are the same as in Example 1 (3).
[0036] (4) The steps are the same as in Example 1 (4).
[0037] (5) The steps are the same as in Example 1 (5).
[0038] (6) The steps are the same as in Example 1 (6).
[0039] (7) The steps are the same as in Example 1 (7).
Embodiment 3
[0041] (1) The steps are the same as in Example 1 (1).
[0042] (2) Dissolve taurochenodeoxycholic acid (10.0g, 18.5mmol) in 50ml of acetone aqueous solution (acetone (V) / water (V)=4 / 1), and slowly add in batches after the dissolution is complete. Chloroperoxybenzoic acid (80%, 6.5g, 30mmol), stirred at room temperature for 4h; sampled on TLC plate, and the reaction was completed after the disappearance of the raw material point, the acetone was evaporated under reduced pressure, extracted with ethyl acetate, the organic layer was separated, and the aqueous phase was Concentrate to dryness to obtain a crude product, which is further recrystallized from a mixed solvent of water / acetone (1:10 (v:v)) to obtain 7.0 g of 3α-hydroxy-7-carbonyl-cholanoyl-N-taurine.
[0043] (3) The steps are the same as in Example 1 (3).
[0044] (4) The steps are the same as in Example 1 (4).
[0045] (5) The steps are the same as in Example 1 (5).
[0046] (6) The steps are the same as in Exampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com