Application of colloidal gold to preparation of artificial cerebrospinal fluid reagent
A technology of artificial cerebrospinal fluid and colloidal gold, which is applied in the field of medical artificial cerebrospinal fluid preparation, can solve the problems of disrupting the circulation stability of cerebrospinal fluid, nerve cell edema and degeneration, etc., and achieve the effect of convenient and quick operation, simple raw materials, and improvement of cerebral edema
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A preparation method of an artificial cerebrospinal fluid preparation containing colloidal gold, comprising the following steps: at 25° C., dissolving chloroauric acid crystals in distilled water to prepare 100 ml of chloroauric acid solution with a concentration of 0.01%, heating to 95° C., maintaining 15 minutes; Add the 0.1% trisodium citrate aqueous solution of 25 μ l rapidly respectively, stir while heating for 30 minutes, stirring speed is set at 200 rev / min, obtains colloidal gold solution; Centrifuge at ℃, discard the supernatant, dissolve with 100ml of distilled water and centrifuge at 8000 rpm at 4℃, repeat three times. Then add 0.6279g of sodium chloride, 0.0216g of potassium chloride, 0.0353g of calcium chloride, 0.1932g of sodium bicarbonate, 0.0488g of magnesium chloride, 0.06g of glucose, and 0.0358g of disodium hydrogen phosphate. Finally, dilute to 100ml with double distilled water.
[0027] Configured artificial cerebrospinal fluid containing 95% O 2...
Embodiment 2
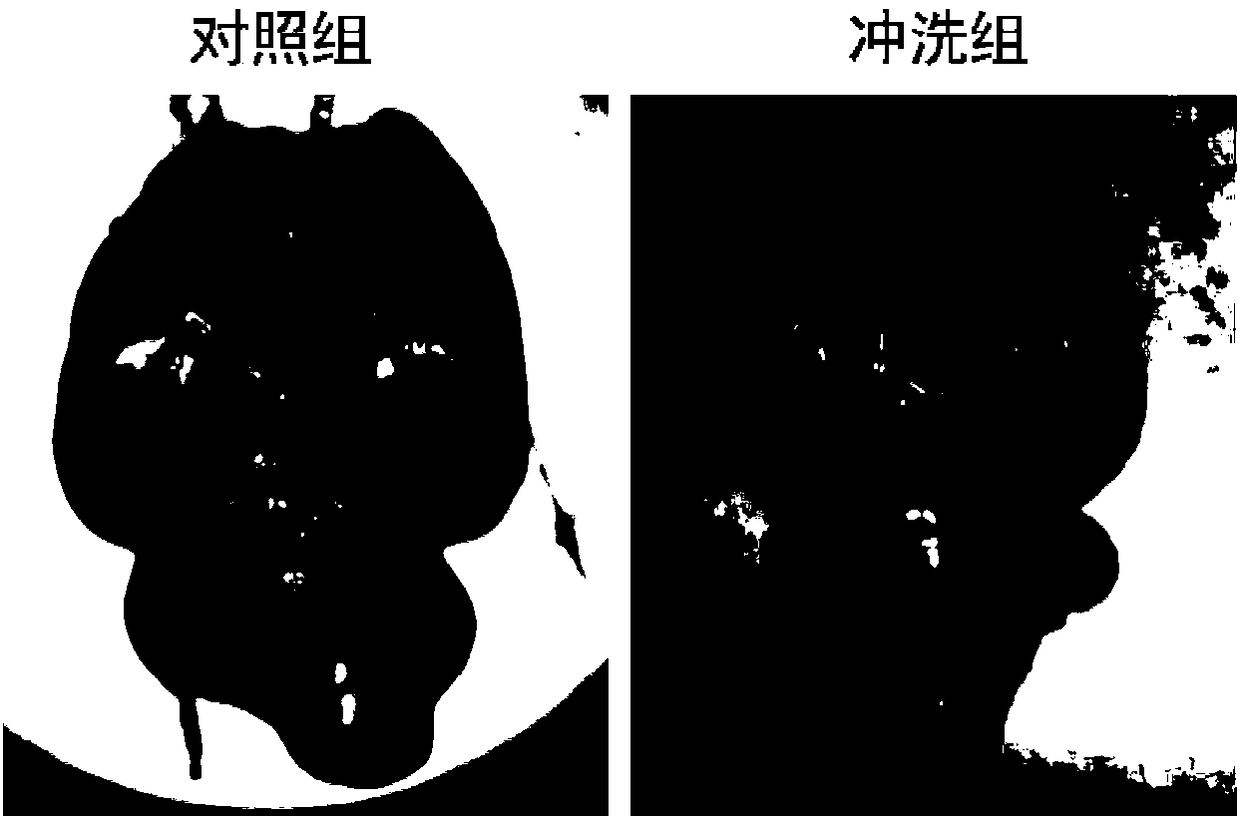
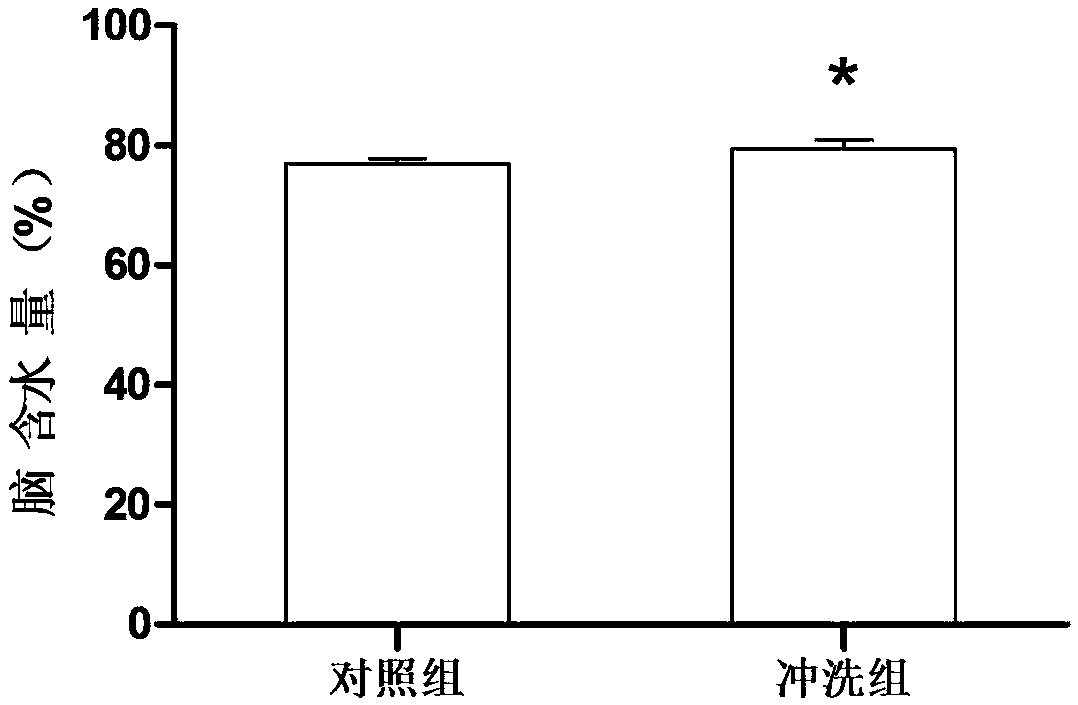
[0029] Male Sprague-Dawley rats (body weight 250-300g) with experimental subarachnoid hemorrhage were randomly divided into artificial cerebrospinal fluid flushing group (n=12) and control group (n=12) containing colloidal gold. After entering the subarachnoid hemorrhage, the cerebrospinal fluid was flushed for 24 hours, specifically: the artificial cerebrospinal fluid containing colloidal gold was slowly pumped into the lateral ventricle (at a rate of 0.5ml / h), and the lumbar cistern was drained. On the 7th day after the subarachnoid hemorrhage, the animals in the two groups were sacrificed to observe the changes in the amount of subarachnoid hematoma in the skull base and the water content in the brain (detection index of cerebral edema). The experimental results are as follows: after dissecting the brain tissue of the rats, the general observation found that the volume of skull base hematoma in the new artificial cerebrospinal fluid flushing group was significantly less than...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com