Sterilizing agent containing 1, 3, 4-oxadiazole cyclodistyrene amide as well as preparation method and application thereof
A technology of oxadiazole ring stilbene amide and fungicide, which is applied in the field of 1,3,4-oxadiazole ring stilbene amide compound and its preparation, and can solve the problems of difficult removal of by-products, high temperature, and resistance to Sexual problems and other problems, to achieve a good effect of prevention and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of (E)-4-(4-(2-thienyl)-1,3,4-oxadiazole)styryl)benzoic acid methyl ester
[0019]
[0020] Dissolve 1.13 g (3.0 mmol) of 4-(5-thienyl)-1,3,4-oxadiazol-2-yl)benzyl diethyl phosphate and 3.0 mmol of methyl p-formylbenzoate in 20 mL In N,N-dimethylformamide (DMF), after half an hour of reaction at room temperature, slowly add 10 mL of ethanol solution containing 0.41 g (3.6 mmol) of potassium tert-butoxide (t-BuOK) dropwise, and continue the reaction at room temperature for 6 hours; Add 20 mL of distilled water to the mixture, cool, filter and wash, dry by infrared, and recrystallize the crude product from a mixed solvent of dimethyl sulfoxide (DMSO) / water to obtain a light yellow solid (E)-4-(4-(2-thienyl) -1,3,4-oxadiazole) styryl) methyl benzoate, yield 79%.
Embodiment 2
[0022] Preparation of (E)-4-(4-(2-thienyl)-1,3,4-oxadiazole)styryl)benzoic acid
[0023]
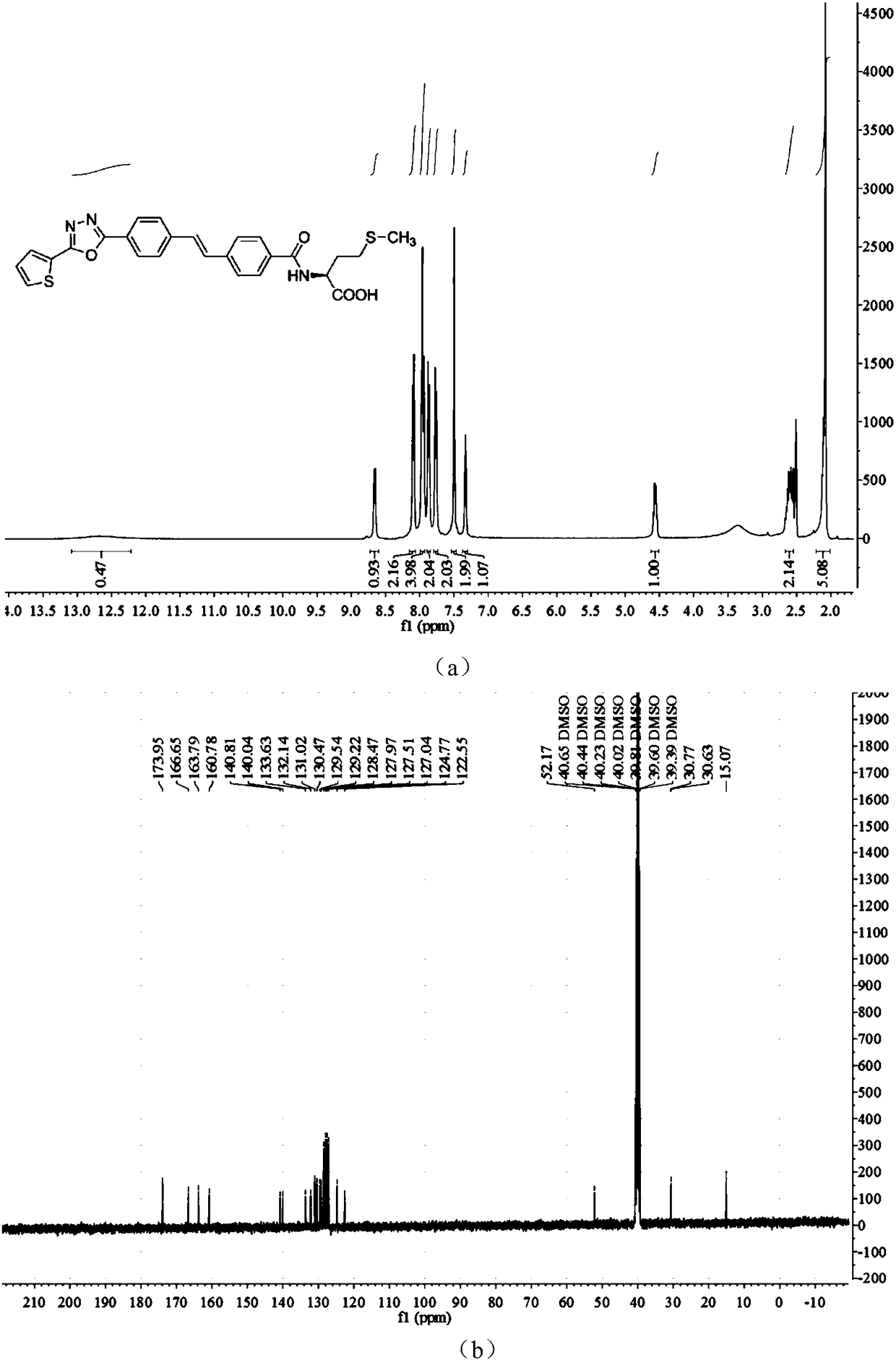
[0024] Add 3mmol (E)-methyl 4-(4-(2-thienyl)-1,3,4-oxadiazole)styryl)benzoate into 40mL of 1M aqueous sodium hydroxide solution, stir and reflux at 80°C. After the reaction was complete, 10 mL of 1M hydrochloric acid aqueous solution was added, the reaction system was extracted twice with ethyl acetate, and the resulting organic layer was washed with anhydrous Na 2 SO 4 After drying, the yellow solid (E)-4-(4-(2-thienyl)-1,3,4-oxadiazole)styryl)benzoic acid was obtained in a yield of 75.4%. Melting point(mp), 333-335℃; 1 HNMR (400MHz, DMSO-d6) δ12.91 (s, COOH, 1H), 8.07 (d, J=8.0Hz, Th-H, 2H), 7.96 (d, J=7.5Hz, Ph-H, 4H) , 7.85(d, J=8.1Hz, Ph-H, 2H), 7.75(d, J=8.0Hz, Ph-H, 2H), 7.48(s, CH=CH, 2H), 7.32(t, J= 4.3Hz, Th-H, 1H); 13 C NMR (101MHz, DMSO-d6) δ167.46, 163.76, 160.78, 141.38, 140.65, 132.12, 131.00, 130.45, 130.29, 130.24, 130.18, 129.20, 128.05, 127.49, 127.267, 124.7 ...
Embodiment 3
[0026] Preparation of (E)-(4-(2-thienyl)-1,3,4-oxadiazol-2-yl)styryl)benzoyl)-L-methionine
[0027]
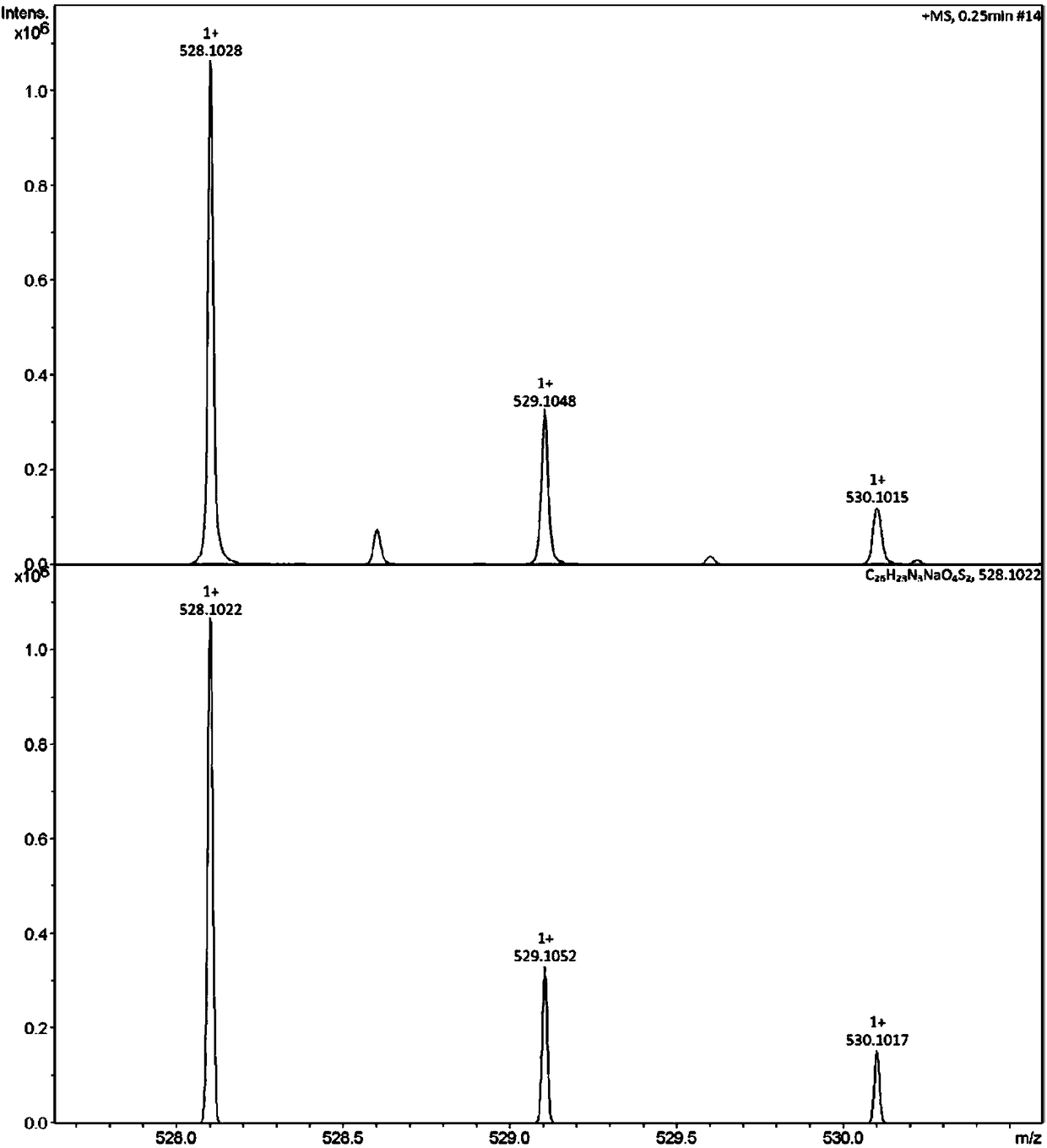
[0028] Dissolve 0.374g (1mmol) (E)-4-(4-(2-thienyl)-1,3,4-oxadiazole)styryl)benzoic acid in 15mL DMF, add 0.135g (1mmol ) 1-hydroxybenzotriazole (HOBt), 0.192g (1mmol) 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDCI) reacted at room temperature for 2 hours and then added 1mmol L-formazol Thionine, stirred at room temperature for 6 hours. After the reaction was completed, 30 mL of distilled water was added, filtered with suction, and washed with 0.5 M hydrochloric acid solution / methanol. The crude product was recrystallized from a mixed solvent of absolute ethanol / dimethyl sulfoxide (DMSO) to obtain (E)-(4-(2-thienyl)-1,3,4-oxadiazol-2-yl as a yellow solid ) styryl) benzoyl) -L-methionine, yield 61.5%. Melting point(mp), 228-230℃; 1 H NMR (400MHz, DMSO-d 6 )δ12.68 (s, COOH, 0H), 8.66 (d, J = 7.7Hz, CONH, 1H), 8.09 (d, J = 8.0Hz, Th-H, 2H), 7.99–7.93 (m, Ph- H, 4H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com