Polysubstituted purine derivatives as well as preparation method and application thereof
A technology of purines and derivatives, applied in the medical field, can solve unseen problems and achieve the effect of reducing by-products, complete reaction, and high antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
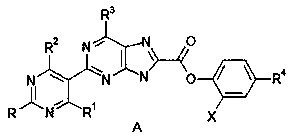
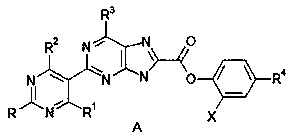
[0040] Synthesis of [2-[(2-nitro-4-hydroxy-5-yl)pyrimidine]-4-pyridine]purin-8-yl-carboxylic acid (D).
[0041] Structural formula:
[0042] .
[0043] (2-Chloro-6-pyridine)purin-8-ylcarboxylic acid (B) (5g, 1.8mol) and (2-nitro-4-hydroxy)pyrimidin-5-yl-boronic acid dimethyl ester (C) ( 5.8g, 2.3mol) into the reaction vessel, in the presence of the organic solvent dichloromethane (50ml) and sodium carbonate (0.8g), add the catalyst 1,1'-bis(diphenylphosphino)ferrocene chloride Palladium (0.32g), heated to 85°C in a water bath and kept stirring, TLC detected that the reaction was complete, generating [2-[(2-nitro-4-hydroxy-5-yl)pyrimidine]-4-pyridine]purine-8- Dimethyl-formic acid (D) (9.69g, 2.4mol).
[0044] Synthesis of [2-[(2-nitro-4-hydroxy-5-yl)pyrimidine]-4-pyridine]purin-8-yl-formyl chloride (E).
[0045] Structural formula:
[0046] .
[0047] The [2-[(2-nitro-4-hydroxy-5-yl)pyrimidine]-4-pyridine]purin-8-yl-formic acid (D) (9.69g, 2.4mol) generated above was...
Embodiment 2-4
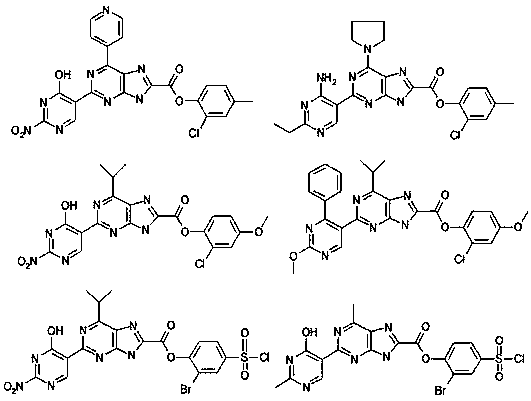
[0054] The catalyst in Example 1 was changed, and other operations were unchanged, and the operation steps of Example 1 were repeated to obtain Examples 2-4, and the results are shown in the following table.
[0055]
[0056] In conjunction with embodiment 1 and embodiment 2~4, catalyzer has important influence to the synthesis of the present invention, uses 1,1'-two (diphenylphosphino) ferrocene palladium chloride to make catalyst and has good catalytic effect, does not Using a catalyst or using palladium as a catalyst or using 1,1'-bis(di-phenylphosphino)ferrocene as a catalyst, the final yield is 10.58% or 60.12% or 48.77%, which seriously affects [2-[( Yield of 2-nitro-4-hydroxy-5-yl)pyrimidin]-4-pyridine]purin-8-yl-formic acid-(2-chloro-4-methyl)phenyl ester (A).
Embodiment 5
[0058] The alkali in Example 1 was removed, and other operations were unchanged, and the operation steps of Example 1 were repeated to obtain Example 5. The results are shown in the table below.
[0059]
[0060] In combination with Example 1 and Example 5, in the absence of alkali, the final [2-[(2-nitro-4-hydroxy-5-yl)pyrimidine]-4-pyridine]purin-8-yl-formic acid-( The yield of 2-chloro-4-methyl)phenyl ester (A) is greatly reduced, so the base plays an important role in the reaction of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com