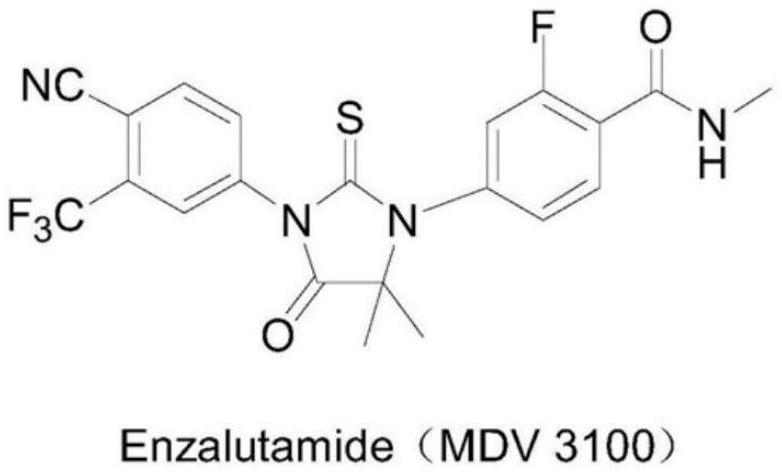
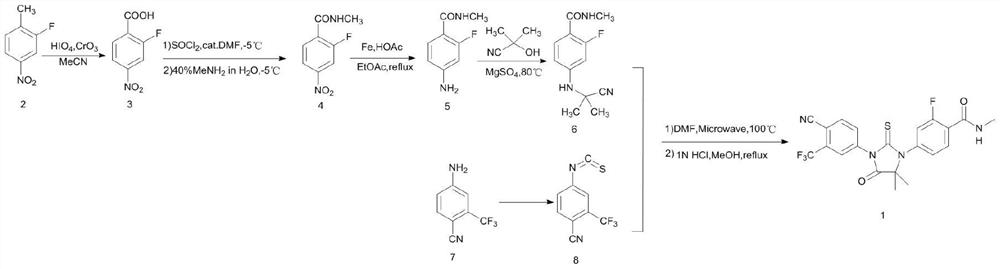
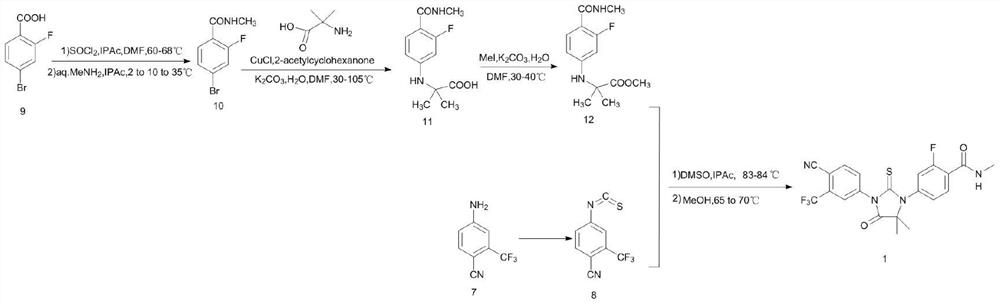
A kind of preparation method of enzalutamide intermediate
A technology of enzalutamide and intermediates, applied in the preparation of carboxylic acid amides, organic compounds, carboxylic acid nitriles, etc., can solve the problems of low synthesis yield and high production cost, and achieve simple process, easy operation, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of compound 15
[0035] Ammonium chloride (9.6g, 0.18mol), propan-2-one (10.2g, 0.17mol), and ammonia (30% aqueous solution, 10mL) were added to 20mL of pure water, and cyanide was added under stirring at 0°C. Sodium chloride (8.6 g, 0.17 mol). The mixed solution was stirred at room temperature for 60 h to stop the reaction, extracted with dichloromethane (2*10 mL), and the combined organic layer was washed with saturated sodium chloride (5 mL) solution, then dried with anhydrous sodium sulfate, filtered, and the filtrate was After concentrating under reduced pressure, a white oil was obtained with a yield of 85-90%.
Embodiment 2
[0036] The preparation of embodiment 2 compound 10
[0037] Compound 9 (2.2g, 10mmol) was added to anhydrous ethylene glycol dimethyl ether (20mL), the solution was cooled to 0°C, and thionyl chloride (1.5g, 13mmol) was slowly added under stirring. The reaction was mild Exothermic, during the addition of thionyl chloride, the solution temperature does not exceed 5°C, after the addition is complete, react at 0°C for 1 hour, and then react the solution at 50°C for no less than 6 hours or TLC monitoring shows that the reaction is complete, and the solution temperature Cool down to 25°C, slowly add a solution of methylamine (0.9g, 30mmol) dissolved in ethylene glycol dimethyl ether (5mL) dropwise, and react the mixed solution at 35°C for 0.5h or stop the reaction after TLC monitoring shows that the reaction is complete , add saturated brine (15mL), extract with ethyl acetate (2*15mL), separate the layers, combine the organic layers, dry over anhydrous sodium sulfate, filter, conce...
Embodiment 3
[0038] The preparation of embodiment 3 compound 10
[0039] To a stirred solution of compound 9 (1.5 g, 6.84 mmol) in DCM (15 mL) was added dropwise oxalyl chloride (3.45 g, 27.39 mmol) at 0°C. After the addition was complete, 2-3 drops of DMF were added at 0 °C and the reaction mixture was stirred at room temperature for 2 h. The reaction mixture was concentrated under reduced pressure and the residue was dissolved in anhydrous THF (20 mL). To this solution was added methylamine (50 mL) at 0°C. The reaction mixture was warmed to room temperature and stirred at room temperature for 30 minutes. The solvent was removed under reduced pressure and the residue was azeotroped with toluene to give the product in 80-85% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com