Method for synthesizing pyrroloquinoline quinone
A technology of pyrroloquinoline quinone and morpholine, which is applied in the field of synthesizing pyrroloquinoline quinone, can solve the problems of difficult raw materials and intermediates, high toxicity, high price, etc., achieve low solubility, increase reaction efficiency, increase reaction speed and Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
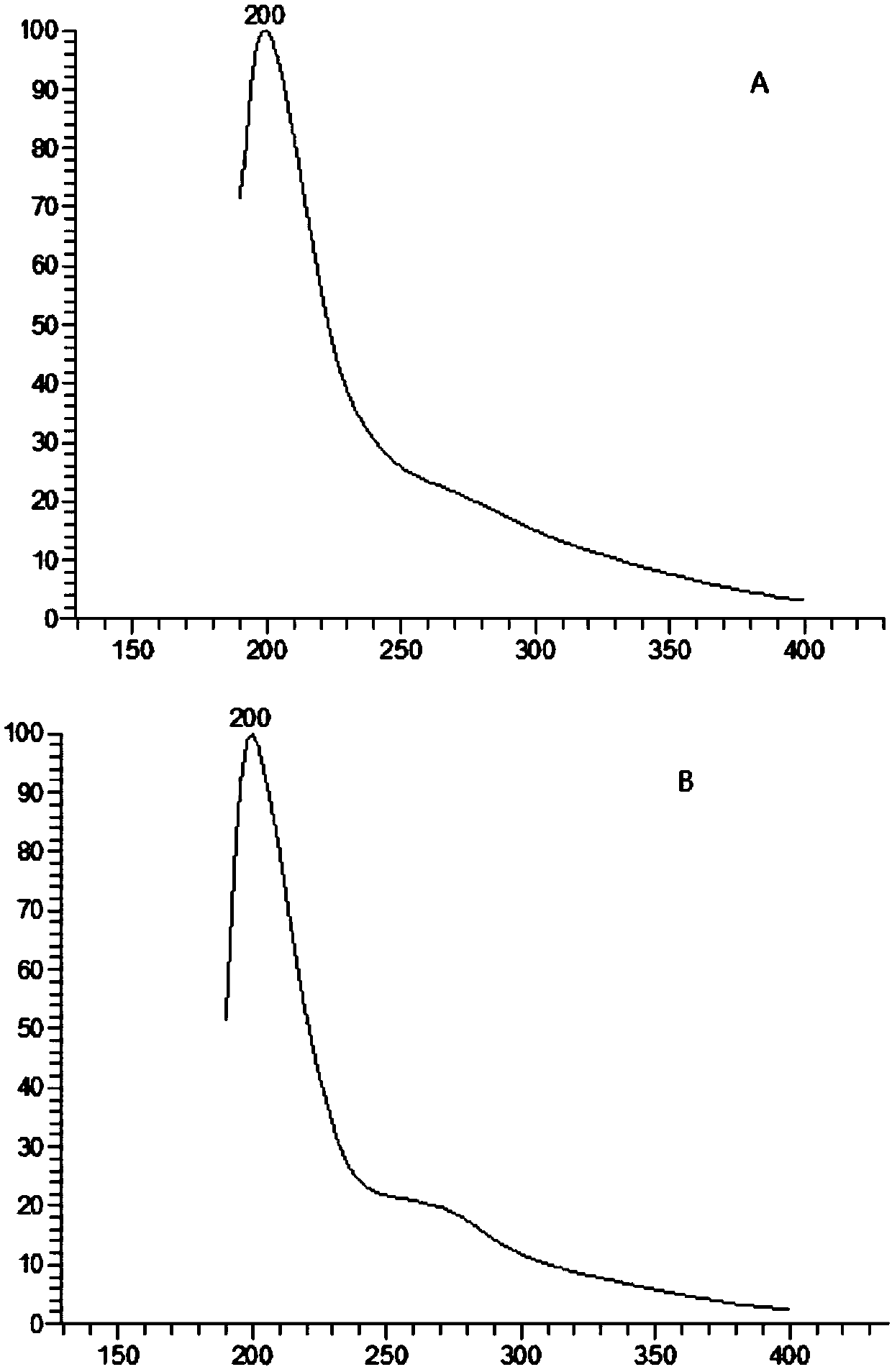
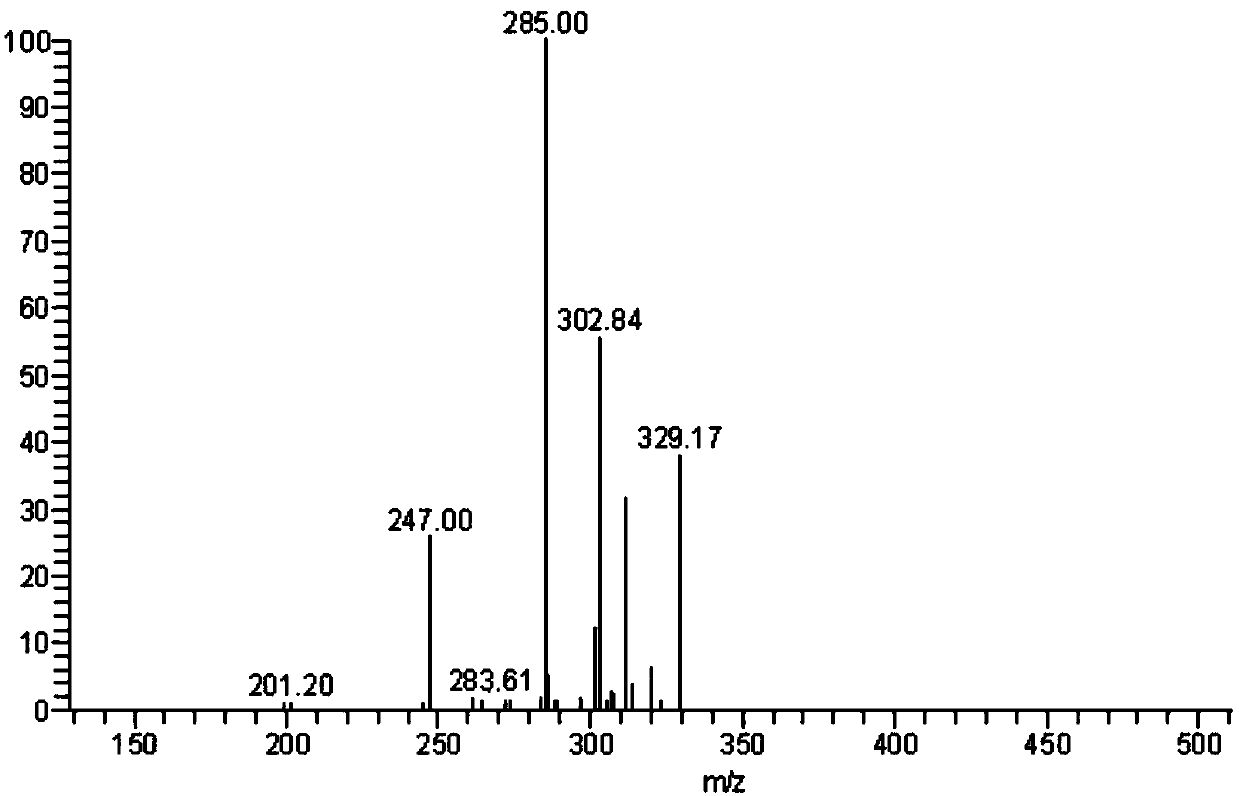
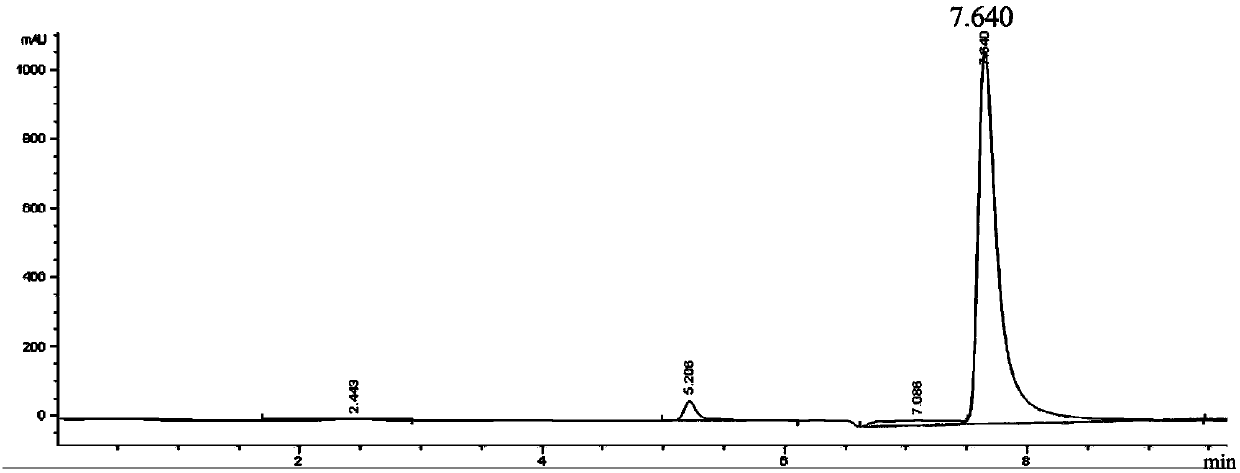
Image
Examples
Embodiment 1
[0041] The method for synthesizing pyrroloquinoline quinone may further comprise the steps:
[0042] 1) Take 116.12g of ethyl pyruvate and 159.81g of bromine water (mass fraction 80%), pass through dry carbon dioxide at 20°C and mix and stir for 2h. The reaction product is extracted with dichloromethane, concentrated and dried under normal pressure to obtain 158.1g of compound 1;
[0043] 2) Take 195g of compound 1 and 69.49g of a saturated aqueous solution of hydroxylamine hydrochloride, mix and stir at 20°C for 10 hours, and extract the reaction product with 5 times the volume of dichloromethane, concentrate and dry under reduced pressure to obtain 181.2g of compound 2;
[0044] 3) Take 210g of compound 2 and 118g of 1,4-cyclohexanedione, add 30mg of morpholine and 60mg of iron dodecacarbonyl as catalysts, and reflux in 500ml of toluene for 4h, and the reaction product is separated and purified by chromatography to obtain 197.2 g Compound 3;
[0045] 4) Take 207g compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com