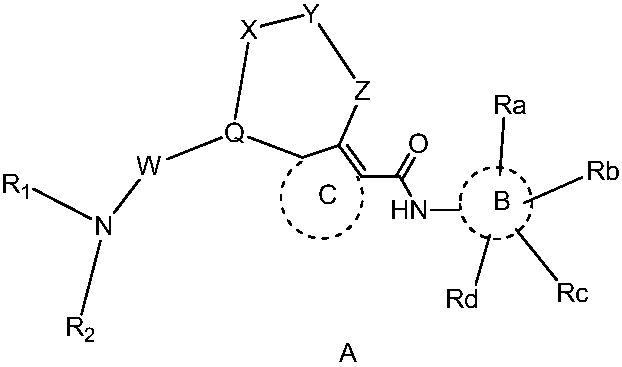
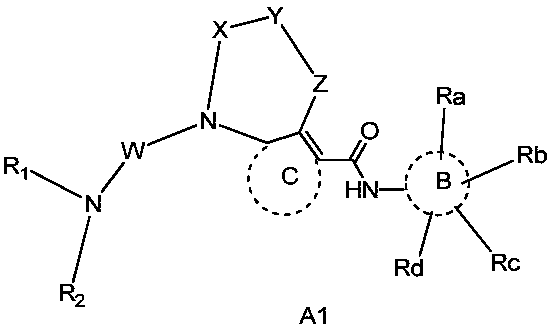
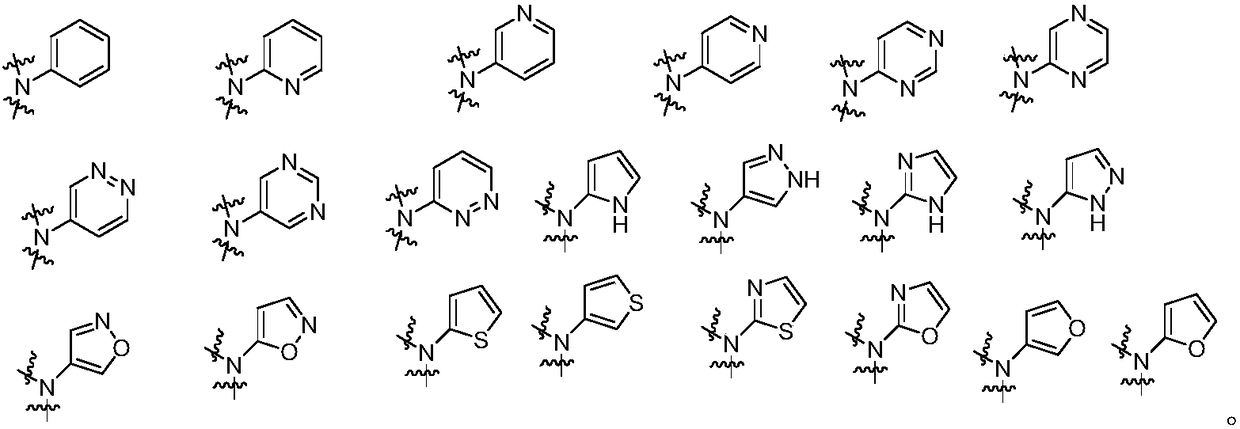
Thiourea and urea compounds and use thereof
A compound and hydrate technology, applied in the field of medicine, can solve problems such as lack of selectivity, poor curative effect, and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Embodiment 1: the synthesis of compound 10a
[0121]
[0122] step 1:
[0123]
[0124] Dissolve compound 1 (1.5g) and propylene bromide (1g) in acetonitrile (15mL), then add cesium carbonate (3.5g) into the reaction system, raise the temperature to 85°C for 2h, add 30(mL) to the reaction system, acetic acid Ethyl ester (3*20mL) was extracted, dried over anhydrous sodium sulfate, the organic phase was spin-dried, and the crude product was chromatographed to obtain compound 2 (1.0g). ESI-MS, (M+H=273.02)
[0125] Step 2:
[0126]
[0127] Compound (1.0 g), ethylene boron anhydride pyridine complex (1.1 g), tetrakistriphenylphosphine palladium (120 mg), potassium carbonate (2.5 g) were dissolved in ethylene glycol dimethyl ether (25 mL) and 4 mL of water, Raise the temperature of the system to 120 degrees under the protection of nitrogen and react for 10 hours, add water (40mL) to the reaction system, extract with ethyl acetate (3*40mL), dry over anhydrous sod...
Embodiment 2
[0140] Embodiment 2: the synthesis of compound 10b
[0141]
[0142] The preparation method of compound 6b refers to steps 1-5 of Example 1, except that in step 4, trifluoromethyl-substituted isopropylsulfonyl chloride is used instead of isopropylsulfonyl chloride.
[0143] According to step 6 of Example 1, only need to replace compound 6 with compound 6b, and keep other conditions unchanged, the target product 10b (12 mg) was obtained by column chromatography (n-heptane:ethyl acetate=1:1).
Embodiment 3
[0144] Embodiment 3: the synthesis of compound 10c
[0145]
[0146] The preparation method of compound 6c refers to steps 1-5 of Example 1, except that in step 4, tert-butylsulfonyl chloride is used instead of isopropylaminesulfonyl chloride.
[0147] According to step 6 of Example 1, only need to replace compound 6 with compound 6c, and keep other conditions unchanged, the target product 10c (22 mg) was obtained by column chromatography (n-heptane: ethyl acetate = 1:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com