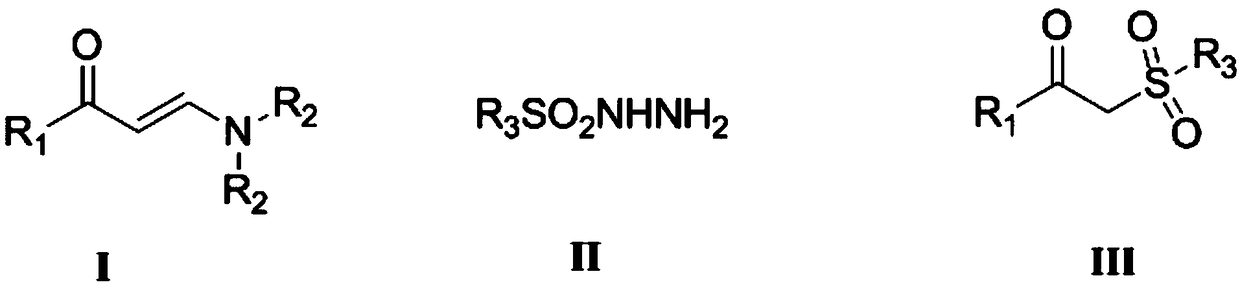
Synthesizing method of beta-keto sulfone compound
A synthesis method and compound technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of excessively demanding reaction equipment and easy production hazards, and achieve high purity and yield. Small volume and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
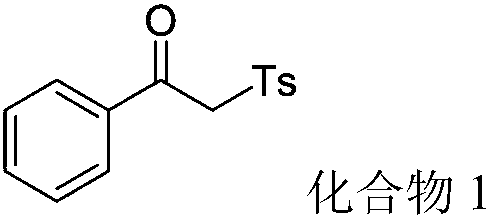
[0046] (1) Step 1: In a dry 100mL round bottom flask, add 1.08g (9mmol) of acetophenone (9mmol) of N,N-dimethylformamide dimethyl acetal 3.9mL (30mmol) and 30mL of xylene as solvent . The mixture was refluxed at 140°C for 12 hours. TLC detects that after the completion of the reaction, the heating is stopped, the solvent is removed under reduced pressure, and the crude product is separated by flash column chromatography to obtain light yellow solid N,N-(dimethylamino)-1-phenylenaminoketone 1.48g (97% yield ).
[0047] (2) Step 2: The above-mentioned N,N-(dimethylamino)-1-phenylenaminone (43.8mg, 0.25mmol), p-toluenesulfonylhydrazide (93mg, 0.5mmol) and tert-butyl Base hydrogen peroxide (64.2mg, 0.5mmol, 70% aqueous solution) was added in the flask, reacted at 80°C for 8 hours, copper acetate (9.08mg, 0.05mmol) was added in three times, and the total catalyst was added at the initial stage of the reaction for the first time 1 / 4 of the amount, the second time add ...
Embodiment 2
[0050]
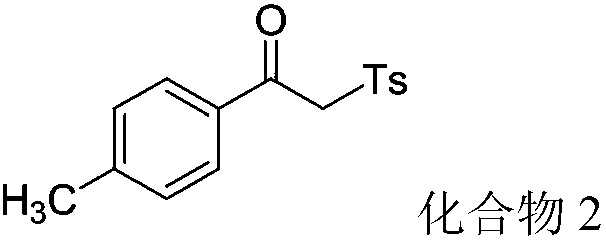
[0051] (1) Step 1: In a dry 100mL round bottom flask, add 1.2g (9mmol) of p-methylacetophenone (9mmol) 3.9mL (30mmol) of N,N-dimethylformamide dimethyl acetal and 30mL of dimethicone Toluene was used as solvent. The mixture was refluxed at 140°C for 12 hours. After the reaction was detected by TLC, the heating was stopped, the solvent was removed under reduced pressure, and the crude product was separated by flash column chromatography to obtain a light yellow solid N,N-(dimethylamino)-1-(4-methylphenyl)enaminone 1.60 g (94% yield).
[0052] (2) Step 2: Combine the above N,N-(dimethylamino)-1-(4-methylphenyl)enaminone (47.3mg, 0.25mmol), p-toluenesulfonylhydrazide (46.5mg , 0.25mmol), and potassium persulfate (96.54mg, 0.25mmol, 70% aqueous solution) were added to the flask, reacted at 40°C for 10 hours, and copper iodide (0.48mg, 0.0025mmol) was added in three times, the first For the first time, 1 / 4 of the total catalyst amount is added at the initial stage of...
Embodiment 3
[0055]
[0056] (1) Step 1: In a dry 100mL round bottom flask, add 1.8g (9mmol) of p-bromoacetophenone, 3.9mL (30mmol) of N,N-dimethylformamide dimethyl acetal and 30mL of dimethicone Toluene was used as solvent. The mixture was refluxed at 140°C for 12 hours. After the reaction was detected by TLC, the heating was stopped, the solvent was removed under reduced pressure, and the crude product was separated by flash column chromatography to obtain 2.15 g of light yellow solid N,N-(dimethylamino)-1-(4-bromophenyl)enaminone (95% yield).
[0057] (2) Step 2: The above-mentioned N,N-(dimethylamino)-1-(4-bromophenyl)enaminone (63.5mg, 0.25mmol), p-toluenesulfonyl hydrazide (232.5mg, 1.25mmol), and benzoyl peroxide (121mg, 0.5mmol) were added to the flask, reacted at 60°C for 8 hours, and copper iodide (2.4mg, 0.0125mmol) was added in three times, the first time at the initial Add 1 / 4 of the total catalyst consumption, add 1 / 2 of the total catalyst consumption in the middle of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com