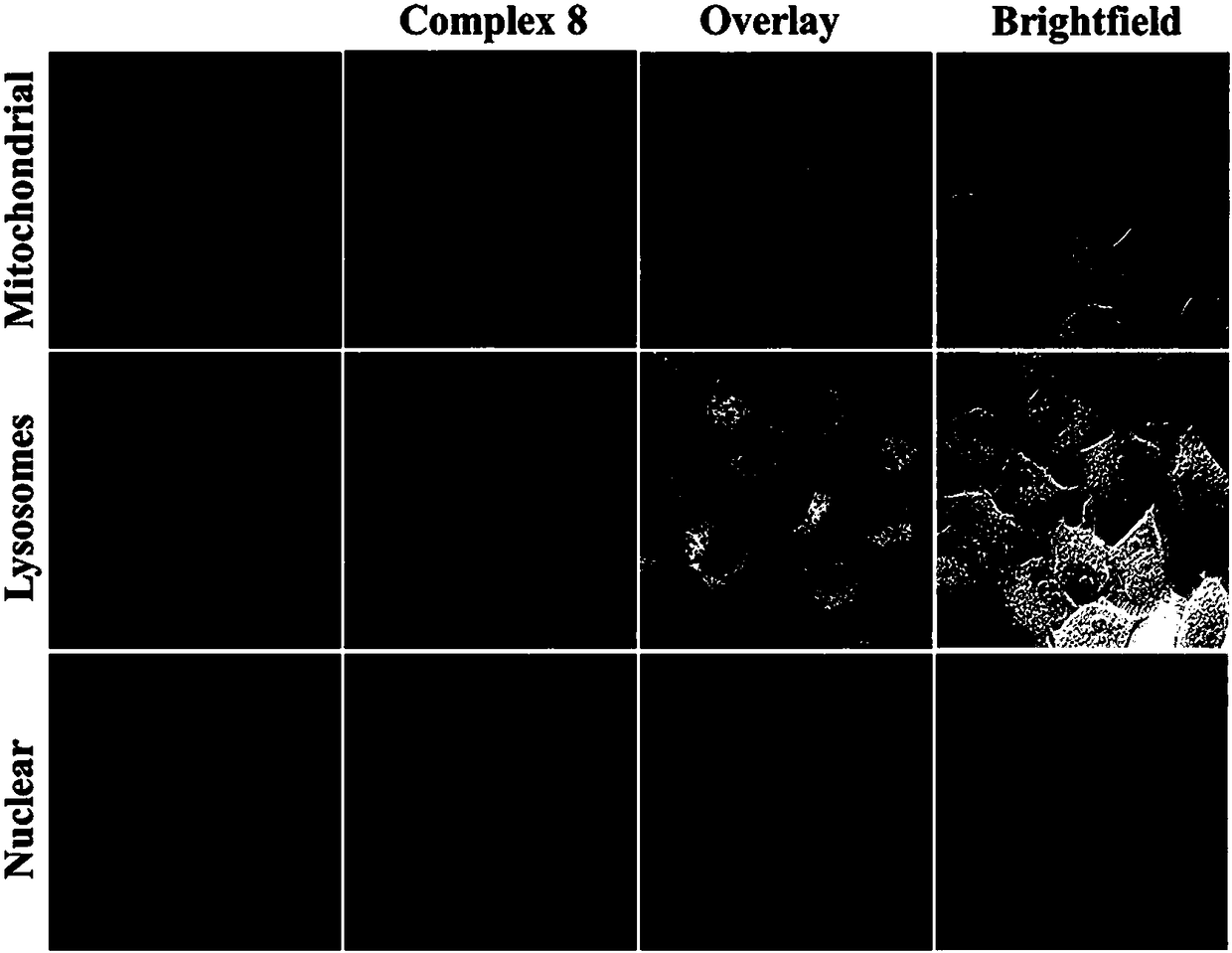
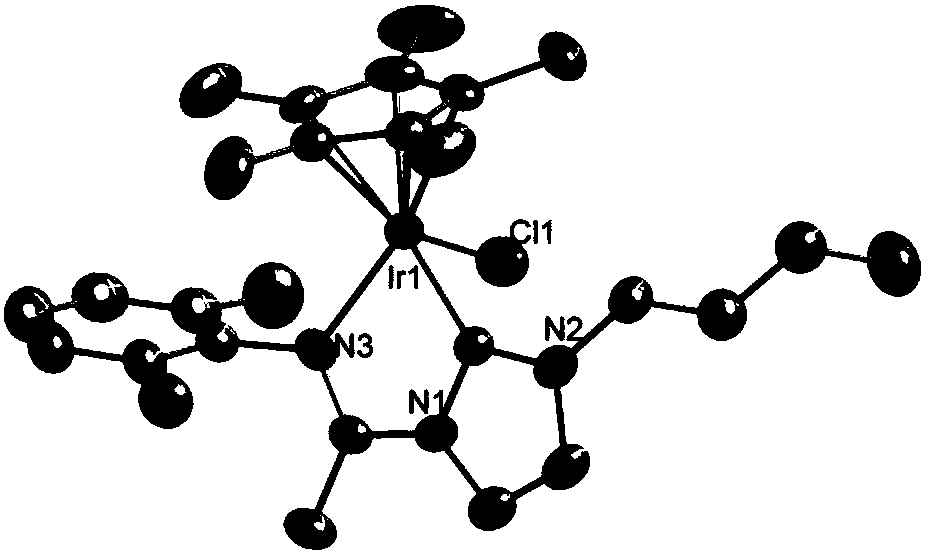
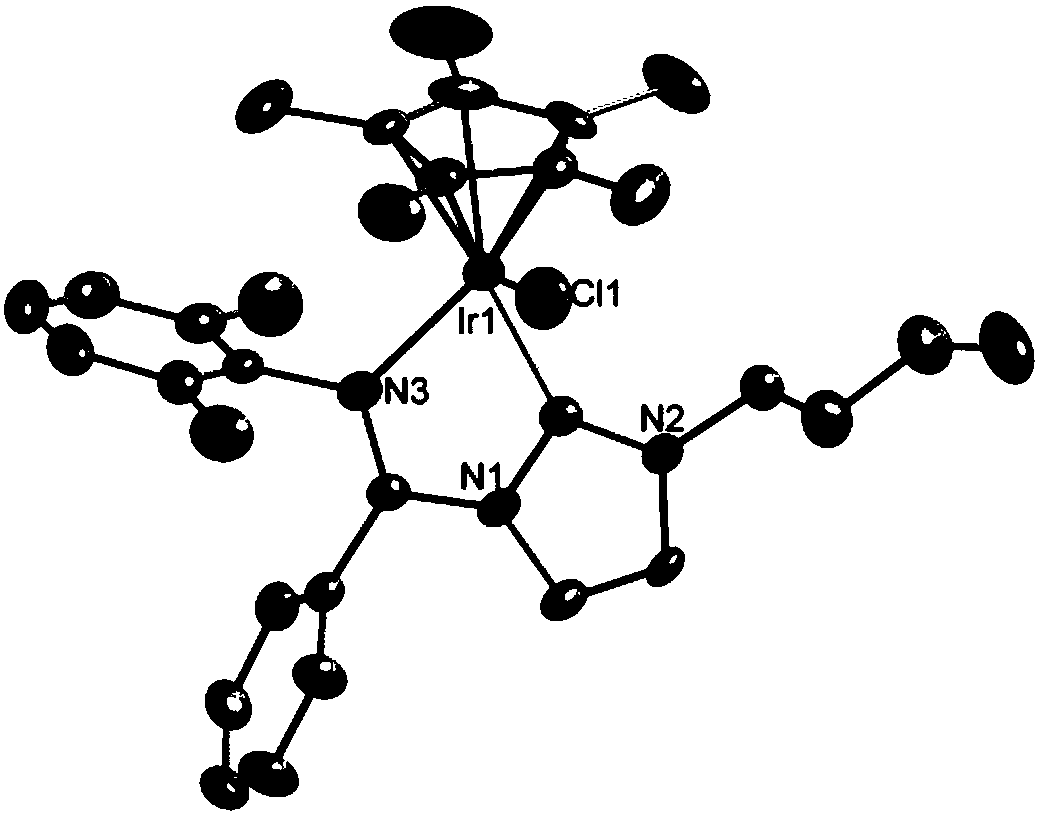
Carbene imine semi-sandwich iridium complex capable of targeting lysosome as well as preparation method and application thereof
A technology of carbene imine and iridium complex, which is applied in the field of chemical pharmaceuticals and can solve problems such as strong cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Weigh 0.5 g IrCl 3 ·nH 2 6 parts of O were added to 6 inner tanks of the microwave digestion apparatus, and 0.75 mL of 1,2,3,4,5-pentamethylcyclopentadiene and 20 mL of methanol were added to each tank, ultrasonicated, nitrogen gas was applied, and the tanks were covered Cover, assemble the main tank and the standard tank, set the parameters of the microwave digestion instrument (the specific parameters are shown in Table 1, the same below), and react with the microwave digestion instrument. The obtained red solid product is dissolved in dichloromethane and filtered through diatomaceous earth. Remove unreacted IrCl 3 ·nH 2 O, after that, recrystallized by dichloromethane / ether diffusion method to obtain orange-red iridium dimer (formula (III) R 1 = methyl) product, yield 53.3%. 1 H NMR (500.13 MHz, CDCl 3 ): δ 1.59 (s, J = 1.4 Hz, 15H).
[0070] Table 1
[0071]
Embodiment 2
[0073] Weigh 0.5 g IrCl 3 ·nH 2 6 parts of O were added to 6 inner tanks of the microwave digestion apparatus respectively, and 1.3 g of 1-biphenyl, 2,3,4,5-tetramethylcyclopentadiene, 20 mL of methanol were added to each tank, ultrasonicated, and nitrogen gas , cover the tank lid, assemble the main tank and the standard tank, set the parameters of the microwave digestion instrument, and react with the microwave digestion instrument. The red solid product obtained is dissolved in dichloromethane and filtered through diatomaceous earth to remove unreacted IrCl 3 ·nH 2 O, after that, recrystallized by dichloromethane / ether diffusion method to obtain orange-red iridium dimer (formula (III) R 1 = biphenyl) product, yield 18.7%. 1 H NMR (500 MHz, DMSO) δ 7.77–7.64 (m,6H), 7.48 (t, J = 7.6 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H), 1.72 (d, J = 14.7Hz , 12H).
Embodiment 3
[0075] 25.0 mg [3-Me-1-(2, 6-dimethylphenyl)iminyl-C 3 h 3 N 2 ] + Cl - and 26.4 mg Ag 2 O was placed in a 25 mL round bottom flask, stirred at room temperature for 6 h in the dark, and then filtered through celite to remove excess Ag 2 O, and wash the filter cake with dichloromethane, and pour the filtrate into a container containing 37.8 mg iridium dimer (formula (III) R 1 = methyl) in a round bottom flask, stirred overnight at room temperature, then added 103.2 mg KPF 6 Continue to stir for 30 min, hang the solvent to dryness with a rotary evaporator to obtain a yellow solid, dissolve the solid with dichloromethane, and filter with a 0.22 μm filter head to remove excess KPF 6 , with CH 2 Cl 2 / n-hexane recrystallization to obtain a yellow crystal product.
[0076] H NMR and mass spectrograms such as image 3 and Figure 15 Shown: 1 H NMR (500 MHz, CDCl 3 ) δ 7.61 (d, J =2.3 Hz, 1H), 7.29 (d, J = 2.3 Hz, 1H), 7.24 (dd, J = 6.2, 2.8 Hz, 1H), 7.22 –7.15 (m, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com