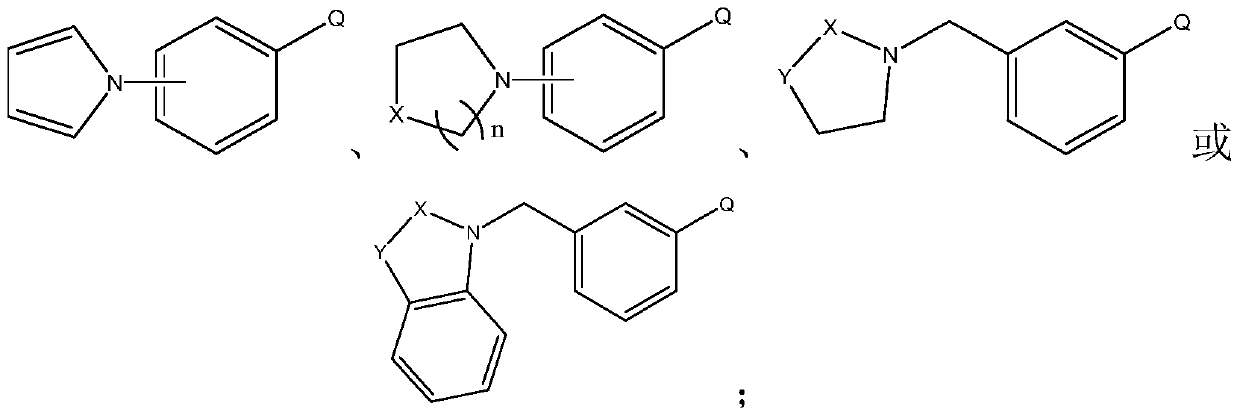
A kind of preparation method of heterocyclic biphenyl boronic acid
A technology of heterocyclic biphenyl boronic acid and cyclic biphenyl boronic acid, which is applied in the field of organic synthesis, can solve the problems of difficult manual manipulation, inability to perform automatic control, and has limitations, and achieves improved operability, stable structure, and overcome low temperature. difficult effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
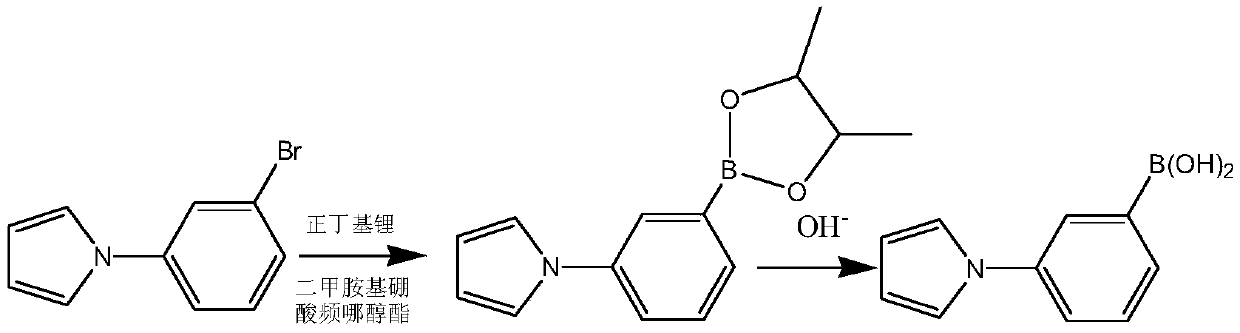
[0036] This embodiment prepares heterocyclic biphenyl boronic acid through the following steps, wherein the compound of formula I has the structure Concrete reaction formula is as follows:
[0037]
[0038] Dissolve 0.01mol of the compound shown in formula I in solvent A tetrahydrofuran, dissolve 0.012mol dimethylaminoboronic acid pinacol ester and 0.012mol n-butyllithium in solvent B tetrahydrofuran, then dissolve solvent A and solvent B in - At 10°C, the continuous flow feeding reaction was carried out for 25 minutes, the reaction was quenched by saturated sodium chloride, after washing, the hydrolysis reaction was carried out with sodium hydroxide at 30°C for 2 hours to obtain 1-(3-phenylboronic acid)-pyrrole, the yield was 90.2 %.
[0039] 1 H NMR (400MHz, DMSO-d 6 )δ7.49-7.08 (m, 4H), 6.62-6.05 (m, 2H), 4.79 (s, 2H).
Embodiment 2
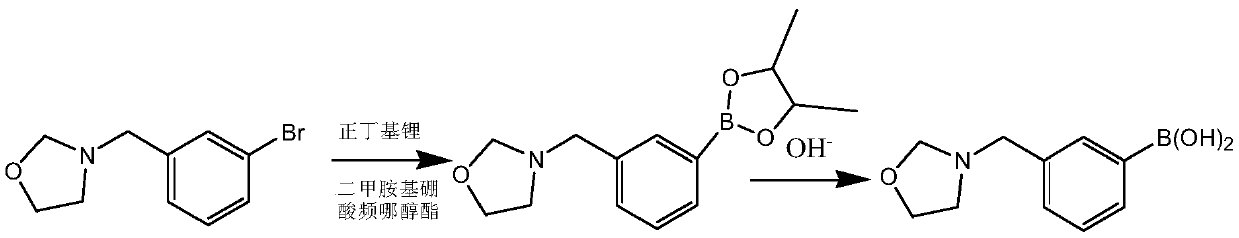
[0041] This embodiment prepares heterocyclic biphenyl boronic acid through the following steps, wherein the compound of formula I has the structure Concrete reaction formula is as follows:
[0042]
[0043] The compound shown in formula I is dissolved in solvent A ether, and dimethylaminoboronic acid pinacol ester and n-butyllithium are dissolved in solvent B ether, wherein the compound shown in formula I, dimethylaminoboronic acid pinacol ester The molar ratio to n-butyllithium is 1:1.1:1.1, and then solvent A and solvent B are subjected to continuous flow feeding reaction at 10°C for 10 min, quenched by saturated sodium chloride, washed, and used potassium hydroxide in The hydrolysis reaction was carried out at 20° C. for 3 h to obtain 3-(3-benzylboronic acid)-oxazolidine with a yield of 88.3%.
[0044] 1 H NMR (400MHz, DMSO-d 6 )δ7.56-7.11 (m, 4H), 4.83 (s, 2H), 4.44 (s, 2H), 3.47 (t, 2H), 2.53 (t, 2H).
Embodiment 3
[0046] This embodiment prepares heterocyclic biphenyl boronic acid through the following steps, wherein the compound of formula I has the structure Concrete reaction formula is as follows:
[0047]
[0048]Dissolve the compound shown in formula I in solvent A tetrahydrofuran, and dissolve dimethylaminoboronic acid pinacol ester and sec-butyllithium in solvent B, wherein the compound shown in formula I, dimethylaminoboronic acid pinacol ester The molar ratio to sec-butyllithium is 1:2.5:2, and then solvent A and solvent B are subjected to continuous flow feeding reaction at -20°C for 60 minutes, and the reaction is quenched by saturated sodium chloride. After washing, use alkaline The reagent was hydrolyzed at 40° C. for 1 h to obtain 1-(3-benzylboronic acid)-1H-indazole with a yield of 87.2%.
[0049] 1 H NMR (400MHz, DMSO-d 6 )δ8.20-8.14 (m, 1H), 7.91-7.03 (m, 8H), 4.99 (s, 2H), 4.22 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com