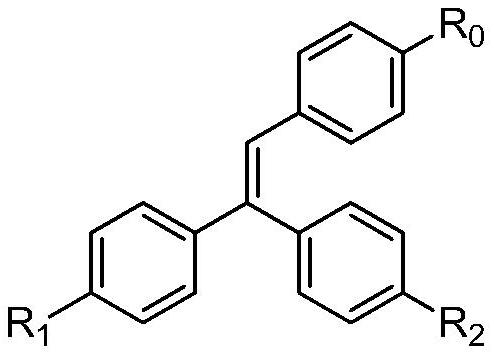
Trihalogen substituted triphenylethylene photochromic material and its synthesis method and application
A technology of photochromic materials and triphenylene, which is applied in the fields of color-changing fluorescent materials, chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, etc. Limit the application prospects of high-speed information storage and other issues, and achieve the effects of easy and easy purification after post-processing, hydrophobic performance, and fast color-changing response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of intermediate [diethyl 4-fluorophenylphosphate]
[0032]
[0033] 2.51 g of 4-fluorobenzyl chloride (0.01 mol) and 6.85 mL of triethyl phosphite (0.04 mol) were added to a 50 ml one-necked flask. Heat to 190°C and stir. After refluxing for 6 h, the reaction was cooled to room temperature, and excess triethyl phosphite was distilled off under reduced pressure. After cooling, n-hexane was added to the system to produce a white precipitate which was filtered and dried to obtain 2.21 g of a white solid with a yield of 90%. (2) synthesis of target product embodiment 1
[0034]
[0035] Add 4,4'-difluorobenzophenone (0.502g, 0.002mol), diethyl 4-fluorophenylphosphate (0.456g, 0.002mol) and dry THF solvent (30mL) into a 100mL three-neck round bottom flask, Stir under the protection of argon atmosphere and cool to 0°C with an ice bath, add potassium tert-butoxide (0.224 g, 0.002 mol), keep the ice bath for 0.5 h, then rise to room temperature and contin...
Embodiment 2
[0038] (1) Synthesis of intermediate [diethyl 4-chlorophenylphosphate]
[0039]
[0040] Referring to the step (1) of Example 1, 4-chlorobenzyl chloride was used instead of 4-fluorobenzyl chloride to synthesize diethyl 4-chlorophenylphosphate, and the yield was 93%.
[0041] (2) synthesis of target product embodiment 2
[0042]
[0043] Referring to step (2) of Example 1, use 4,4'-dichlorobenzophenone to replace 4,4'-difluorobenzophenone, and 4-chlorophenylphosphate diethyl ester to replace 4-fluorophenylphosphate diethyl The target product Example 2 was synthesized with an ester yield of 85%. 1 H NMR (400MHz, Chloroform-d) δ7.32(s,1H),7.30(d,J=2.1Hz,2H),7.27(s,1H),7.20(d,J=8.6Hz,2H),7.15 –7.07(m,4H),6.94(d,J=8.5Hz,2H),6.87(s,1H).
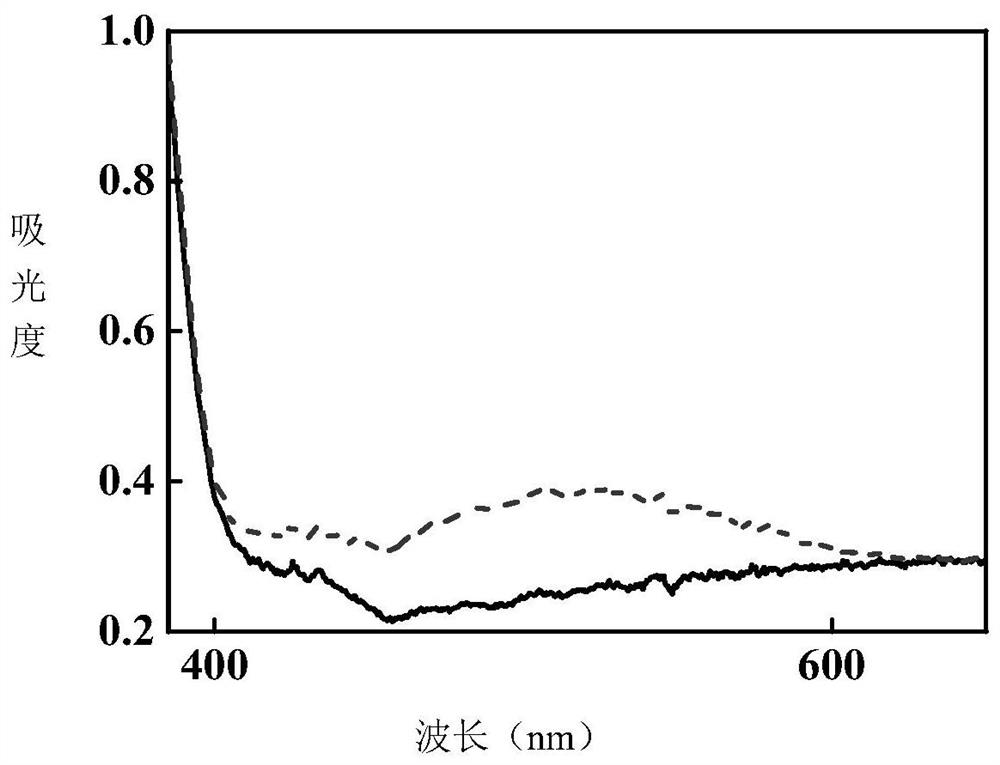
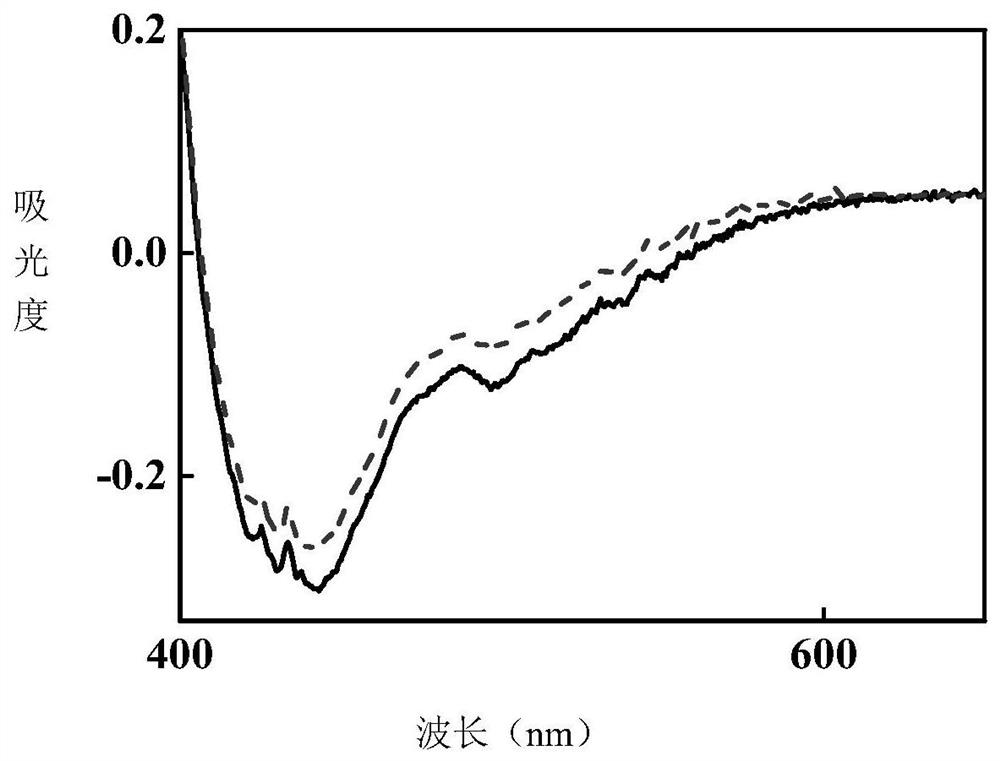
[0044] Under the irradiation of 365nm LED ultraviolet light source, the product of this example gradually turns red from white crystal powder, and the comparison of ultraviolet absorption spectra before and after photochromism is measured ...
Embodiment 3
[0046] (1) Synthesis of intermediate [diethyl 4-iodophenylphosphate]
[0047]
[0048] Referring to the step (1) of Example 1, 4-iodophenyl phosphate diethyl was synthesized by using 4-iodobenzyl bromide instead of 4-fluorobenzyl chloride, and the yield was 81%.
[0049] (2) synthesis of target product embodiment 3
[0050]
[0051] Referring to step (2) of Example 1, 4,4'-dibromobenzophenone is used to replace 4,4'-difluorobenzophenone, and 4-iodophenylphosphate diethyl ester is used to replace 4-fluorophenylphosphate diethyl Ester synthesis target product Example 3, yield 83%. 1 H NMR (400MHz, Chloroform-d) δ7.53–7.41(m,6H),7.14(d,J=8.6Hz,2H),7.03(d,J=8.4Hz,2H),6.84(s,1H) ,6.75(d,J=8.4Hz,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com