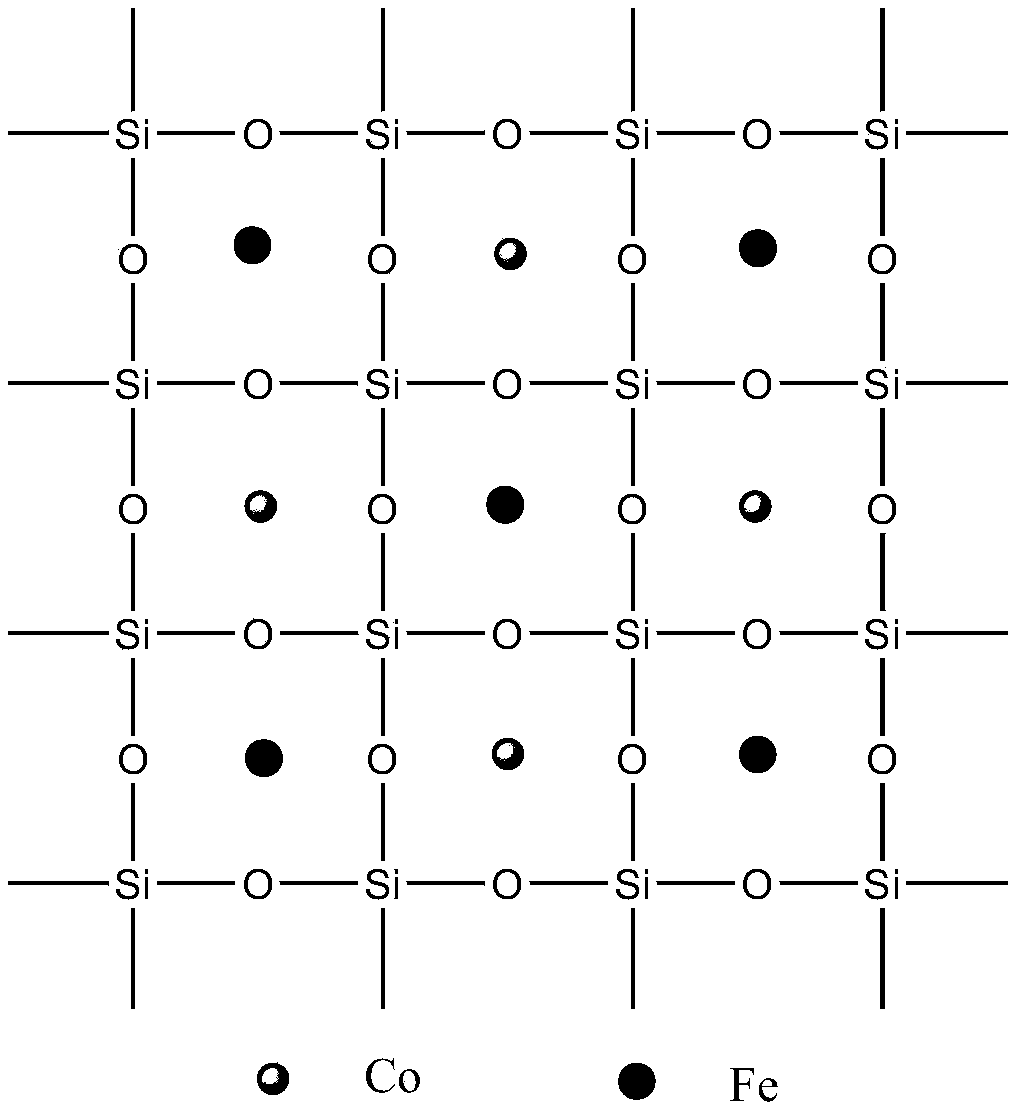
Preparation method of dual-activity monatomic ammonia synthesis catalyst
A catalyst and dual-activity technology, which is applied in the field of preparation of dual-activity single-atom ammonia synthesis catalysts, to achieve the effects of economical and easy-to-obtain raw materials, stable product performance, and convenient methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1) Weigh 4.04gFe(NO 3 ) 3 9H 2 O and 2.38 g CoCl 2 ·6H 2 O, measure 25mL of absolute ethanol in a 50mL beaker ①, stir until dissolved. Take 73mL TEOS and 6.5mL HCl solution into a 200ml beaker ②, the water-to-silicon ratio is about 3, stir evenly; add 2.40g urea into the beaker ②, stir to fully dissolve the urea. Pour the solution in beaker ① into beaker ②, and stir to make it evenly mixed. Pour the mixed solution into a 250mL round bottom flask, adjust the pH of the system to 4.5, heat, stir and reflux at 80-90°C for 2 hours, and place the slightly cooled flask in an incubator at 40°C for 1 day to obtain the initial condensation. glue. The obtained product in the gel state was further vacuum-dried at 60° C. for 1 day to obtain a solid gel.
[0047] 2) Put the product obtained in the above steps into a tube furnace for high-temperature calcination under nitrogen protection at a heating rate of 5°C / min and temperature control at 700°C. After calcination for 8 hour...
Embodiment 2
[0051] 1) Weigh 4.06gFe(NO 3 ) 3 9H 2 O and 2.50 g CuSO 4 ·5H 2 O, measure 25mL of absolute ethanol in a 50ml beaker ①, stir until dissolved. Take 76mL TEOS and 6.8mL HCl solution into a 200ml beaker ②, the water-to-silicon ratio is about 5, stir evenly; add 2.43g urea into the beaker ②, stir to fully dissolve the urea. Pour the solution in beaker ① into beaker ②, and stir to make it evenly mixed. Pour the mixed solution into a 250mL round bottom flask, adjust the pH of the system to 4.0, heat, stir and reflux at 80-90°C for 2 hours, and place the slightly cooled flask in an incubator at 40°C for 1 day to obtain the initial condensation. glue. The obtained product in the gel state was further vacuum-dried at 60° C. for 1 day to obtain a solid gel.
[0052] 2) Put the product obtained in the above steps into a tube furnace for high-temperature calcination under nitrogen protection at a heating rate of 10° C. / min and temperature control at 600° C. After 8 hours of calcina...
Embodiment 3
[0055] 1) Weigh 4.12gFe(NO 3 ) 3 9H 2 O and 2.63g NiSO 4 ·6H 2 O, measure 25mL of absolute ethanol in a 50mL beaker ①, stir until dissolved. Take 50mL TEOS and 6.8mL HCl solution into a 200ml beaker ②, the water-to-silicon ratio is about 15, stir evenly; add 2.5g urea into the beaker ②, stir to fully dissolve the urea. Pour the solution in beaker ① into beaker ②, and stir to make it evenly mixed. Pour the mixed solution into a 250ml round bottom flask, adjust the pH of the system to 5.0, heat, stir and reflux at 80-90°C for 2 hours, place the slightly cooled flask in an incubator at 40°C for 1 day to obtain the initial condensation glue. The obtained product in the gel state was further vacuum-dried at 60° C. for 1 day to obtain a solid gel.
[0056] 2) Put the product obtained in the above steps into a tube furnace for high-temperature calcination under nitrogen protection at a heating rate of 5°C / min, controlled at 600°C, and calcine for 6 hours to obtain a black soli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com