Second infrared window excitation/emission fluorescent dye and preparation method and application thereof
A fluorescent dye, near-infrared technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
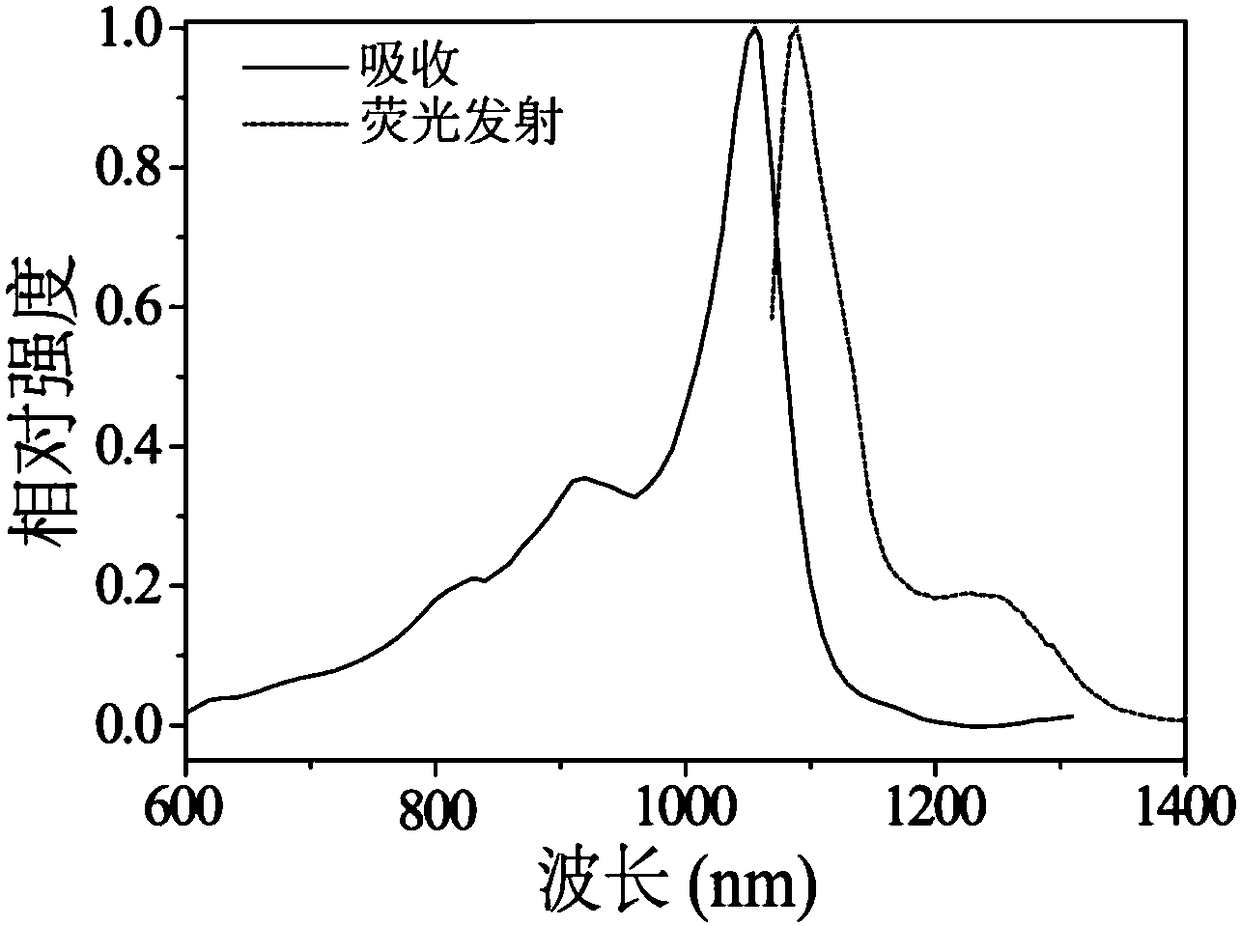
[0033] Preparation of heptamethine fluorescent probe FD-1080. Specific steps are as follows:
[0034] (1) Synthesis of compound 1
[0035] Weigh 1,8-naphtholactam (0.85g, 5mmol) and potassium hydroxide (0.56g, 10mmol) and dissolve in 10mL N-methyl-2-pyrrolidone solution, stir at room temperature for 30min, then add 1,4 -Butane sultone (0.75g, 5.5mmol), the temperature was raised to 90°C, reacted for 10h, cooled to room temperature and then added acetone to obtain a precipitate, which was filtered to obtain compound 1 (1.65g, 96%).
[0036] (2) Synthesis of compound 2
[0037] Weigh compound 1 (1.50g, 5mmol) and tetrabutylammonium chloride (1.51g, 5.5mmol) and dissolve in 8mL acetic acid, react at 90°C for 0.5h, cool to room temperature, add ethyl acetate, filter, spin dry A yellow oily liquid, Compound 2 (2.5 g, 94%) was obtained.
[0038] (3) Synthesis of compound 3
[0039] Weigh compound 2 (2.73g, 5mmol) and dissolve it in 20mL anhydrous tetrahydrofuran (THF), under th...
Embodiment 2
[0043] The method for preparing the complex of heptamethine fluorescent probe FD-1080 and plasma protein, taking fetal bovine serum as an example. Specific steps are as follows:
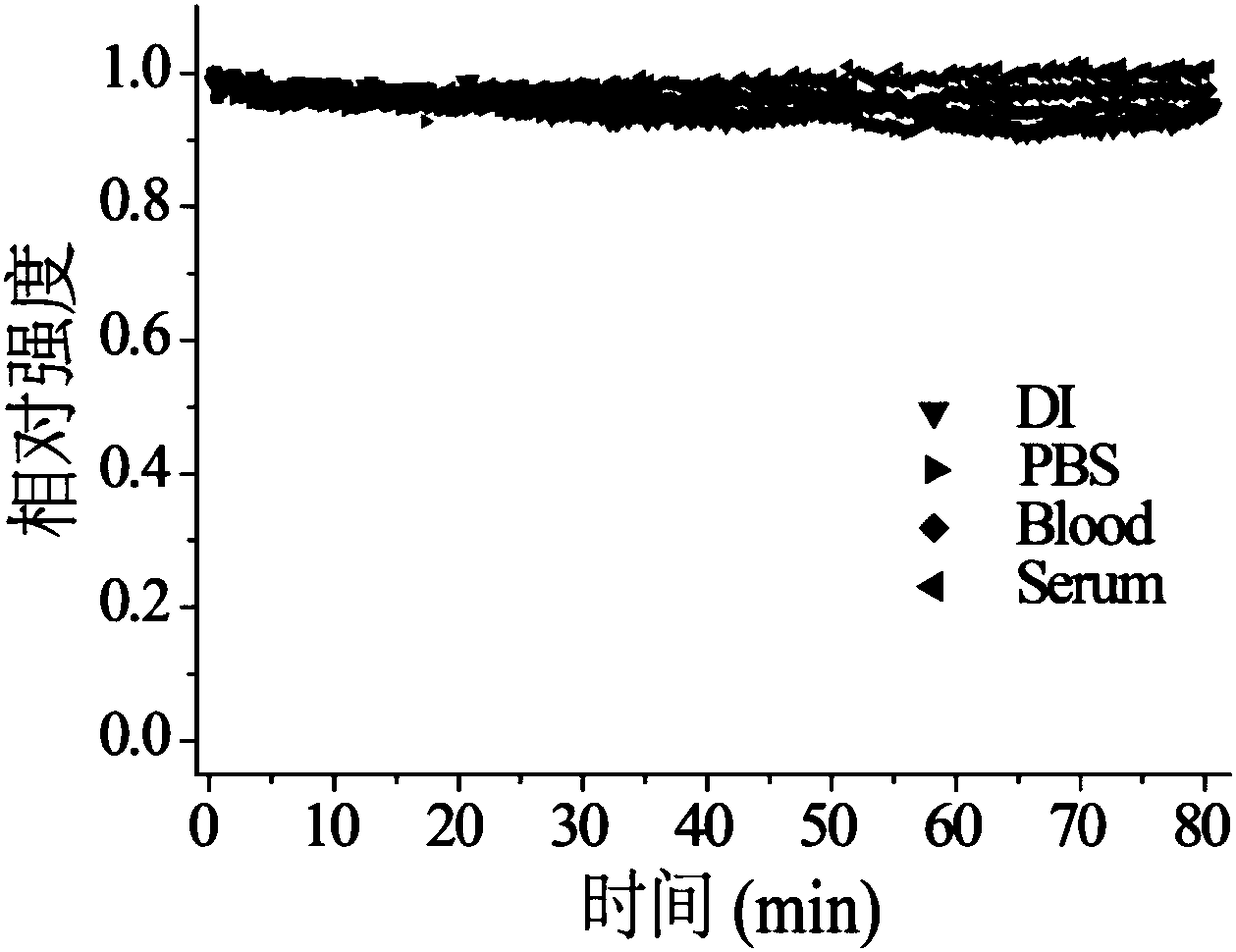
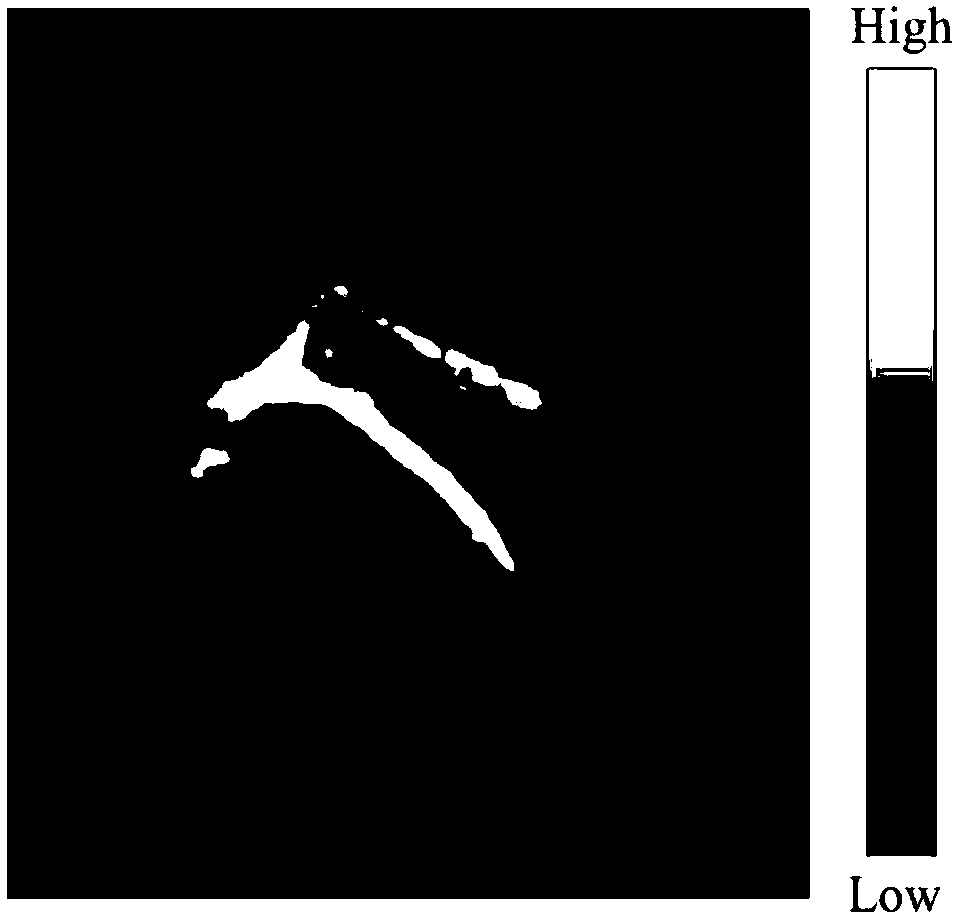
[0044] Prepare the dimethyl sulfoxide solution of dye FD-1080 so that its concentration is 10 -3mol / L, 40 μL was added to 500 μL fetal bovine serum (FBS) solution, and incubated at 40° C. for 110 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com