Method for extracting nickel from chemically-deposited nickel sulfide materials
A chemical precipitation, nickel sulfide technology, applied in the direction of improving process efficiency, can solve the problems of difficult control, sulfur dioxide and hydrogen sulfide gas overflow, and high equipment investment, achieve good comprehensive recycling effect, be beneficial to environmental protection and production operations, and high efficiency. The effect of metal recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
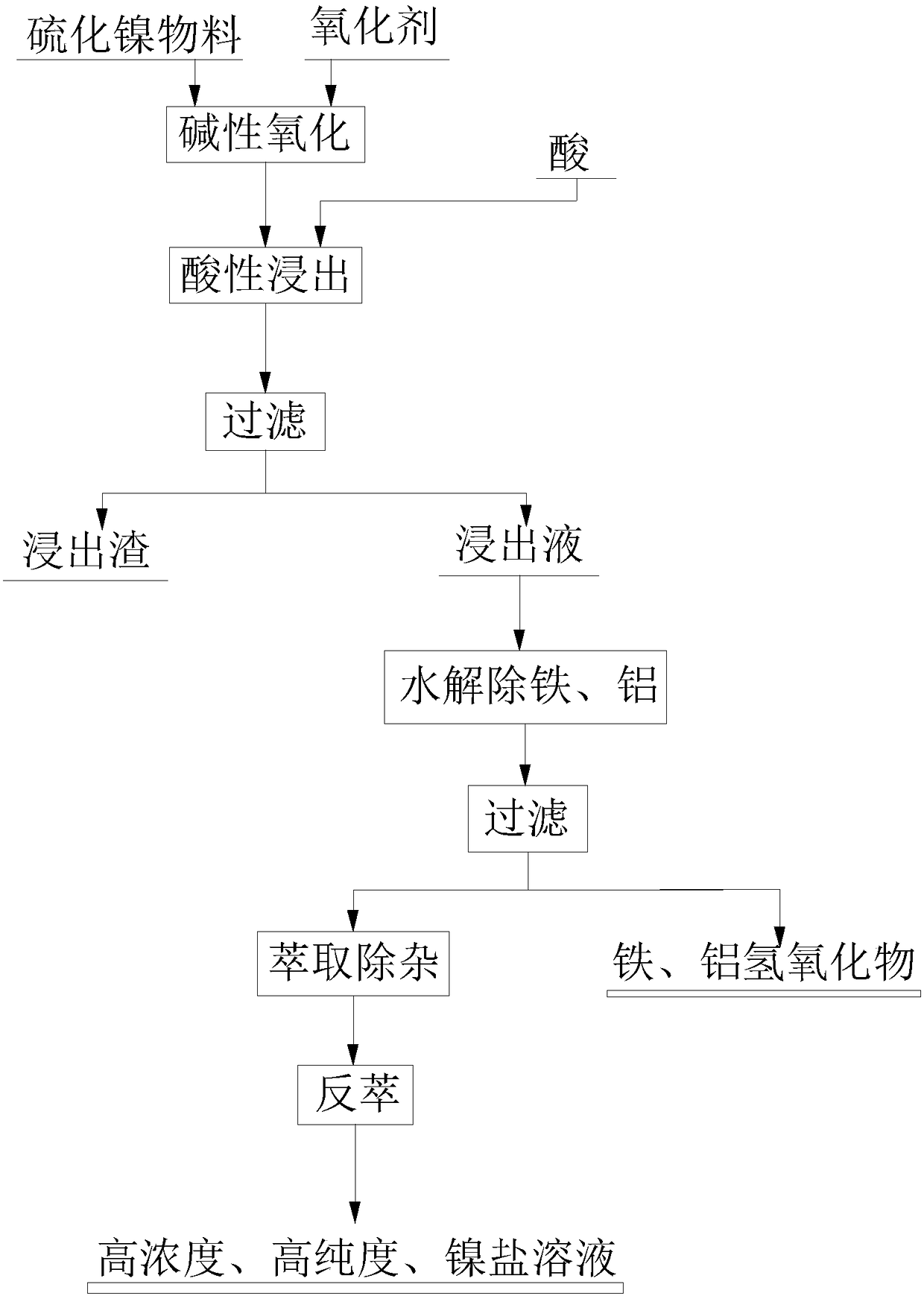
[0033] Put the chemically precipitated nickel sulfide material (containing Ni 48.92%, Co 0.24%, Ca 0.41%, Fe 3.19%, Al 0.02%, S 27.42%) into the reaction tank, add water to adjust the slurry according to the liquid-solid ratio of 4:1, and stir The speed is 400 r / min, and sodium hydroxide is added to adjust its pH to 8.0; The temperature is 30°C, and the reaction time is 5h; after the reaction is completed, the initial hydrogen ion concentration in the reactor is 1.5mol / L after adding sulfuric acid, the leaching temperature is controlled at 80°C, the stirring speed is 400r / min, and the leaching time is 7h; filter, Obtain leaching liquid and leaching slag; return leaching slag to slurry; adjust pH of leaching liquid to 3.5-4.0; after filtering, carry out multi-stage countercurrent extraction and removal of impurities with sodium soap P204 (containing 75% kerosene) extraction system at a ratio of 1:1 , the nickel-loaded organic can be back-extracted with 2mol / L sulfuric acid solu...
Embodiment 2
[0035] Put the chemically precipitated nickel sulfide material (containing Ni 48.92%, Co 0.24%, Ca 0.41%, Fe 3.19%, Al 0.02%, S 27.42%) into the reaction tank, add water to adjust the slurry according to the liquid-solid ratio of 7:1, and stir The speed is 200 r / min, and adding sodium hydroxide is adjusted to its pH to 9.5; 50°C, the reaction time is 9h; after the reaction is completed, the initial hydrogen ion concentration in the reactor is 3.0mol / L after adding hydrochloric acid, the leaching temperature is controlled at 98°C, the stirring speed is 250r / min, and the leaching time is 3h; filter, Obtain leaching solution and leaching slag; Nickel content is 0.08wt% in the detection slag, promptly sends outside slag field unified processing and gets final product; The pH of leaching solution is adjusted to 4.0; After filtering, compare 1:1 with nickel soap P204 (containing 75% kerosene) extraction system for multi-stage countercurrent extraction to remove impurities, nickel-lo...
Embodiment 3
[0037] Put the chemically precipitated nickel sulfide material (containing Ni 51.04%, Co 0.12%, Ca 0.35%, Fe 2.07%, Al 0.15%, S 28.46%) into the reaction tank, add water to adjust the slurry according to the liquid-solid ratio of 5:1, and stir The speed is 300 r / min, and adding sodium hydroxide is adjusted to its pH to 9.0; 40°C, the reaction time is 7h; after the reaction is completed, the initial hydrogen ion concentration in the reactor is 2.5mol / L after adding hydrochloric acid, the leaching temperature is controlled at 90°C, the stirring speed is 300r / min, and the leaching time is 3h; filter, Obtain leaching solution and leaching slag; the nickel content in the slag is detected to be 0.38wt%, just return to slurry; the pH of the leaching solution is adjusted to 4.0; after filtering, compare with nickel soap P204 (containing 75% kerosene) by 1:1 The extraction system carries out multi-stage countercurrent extraction to remove impurities. After the nickel-loaded organic is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com