Application of multifunctional polymer vesicles to preparation of medicine for treating multiple myeloma
A multiple myeloma, polymer technology, applied in the direction of antineoplastic drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of impenetrable immunogenicity, protein instability, short half-life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
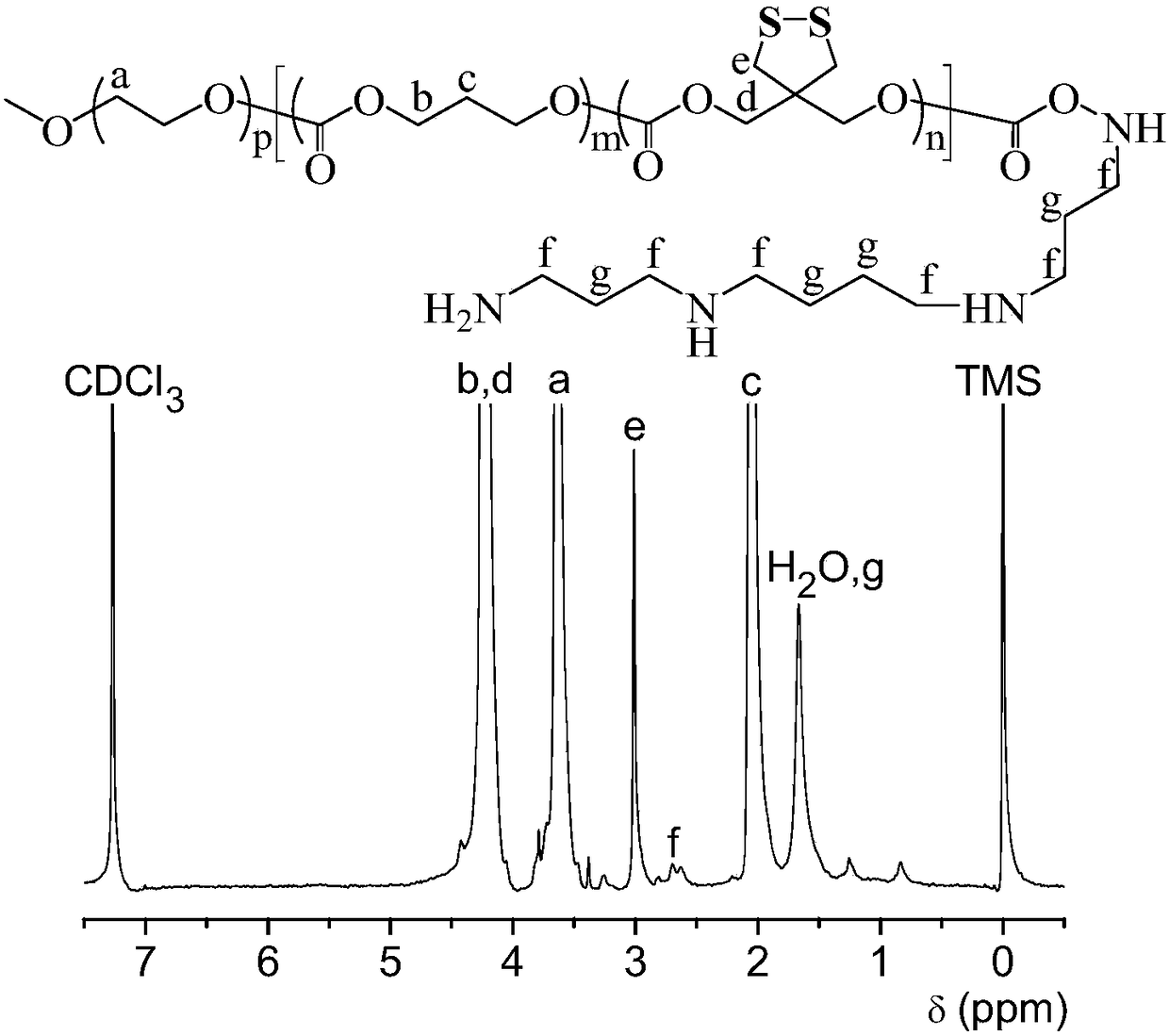
[0034] Example 1 Synthesis of HA-SH and PEG-P(TMC-DTC)-SP Polymers
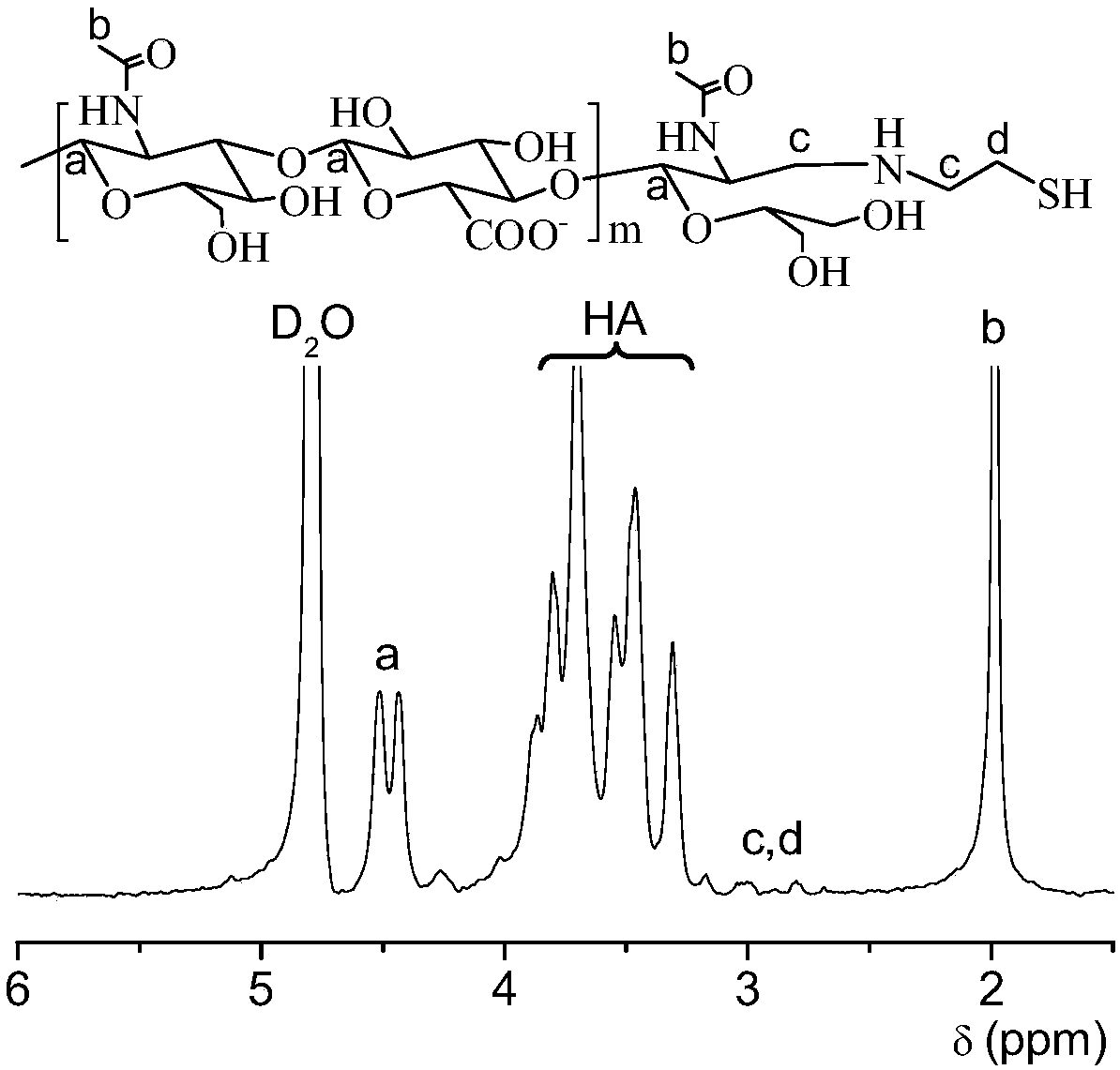
[0035] HA-SH (molecular weight about 17000Da) is obtained from HA through two-step reaction. First, sodium borohydrocyanide (126 mg, 2.0 mmol) was added to a boric acid buffer solution (pH 8.5, 50 mM, 10.0 mL), the entire reaction solution was stirred at 40°C for 5 days. Then dithiothreitol (DTT, 0.15 g, 1.0 mmol) was added to the reaction solution under nitrogen atmosphere, and the reaction was stirred at room temperature for 24 hours. HA-SH was isolated by dialysis in deionized water (MWCO 3500) and freeze-drying under nitrogen protection. Yield: 84%. The conversion rate of HA-SH can be measured to be about 98% by the ELLMAN reagent method. The H NMR spectrum shows that in addition to the signal peaks of HA (δ 1.86, 3.28-4.02, 4.21-4.75), there are new signal peaks at δ 2.68-2.98, which are formed after the aldehyde group at the HA terminal reacts with cystamine The methylene proton peak next to the se...
Embodiment 2
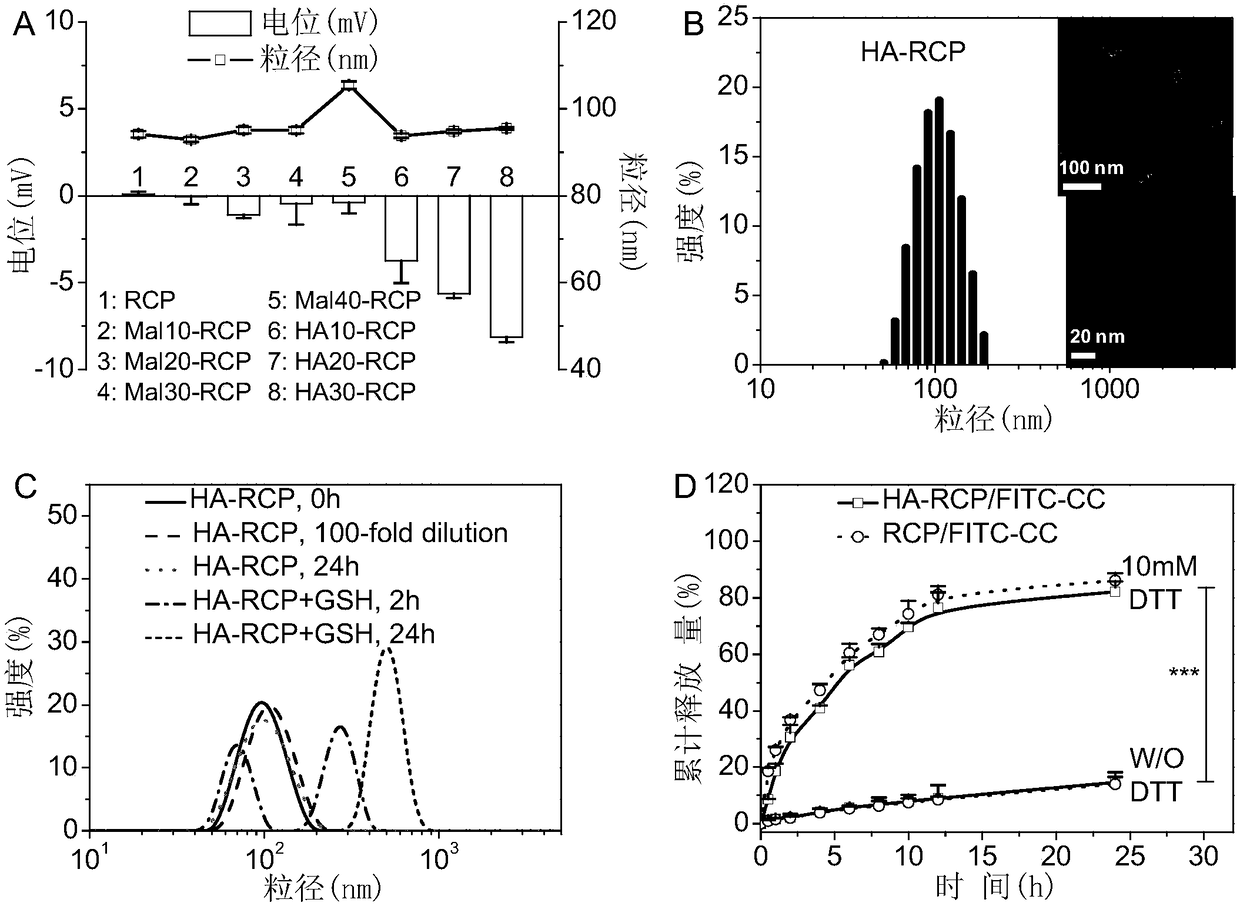
[0044] Example 2 Preparation of post-surface modified HA vesicles (HA-RCP)
[0045] Modified self-crosslinked vesicles after preparation of surface HA for active targeting protein drug delivery. Compared with pre-modified vesicles, HA post-modified vesicles can ensure that HA targeting molecules are fully exposed outside the vesicles to fully interact with CD44 on the surface of tumor cells, and can also ensure that the structure and size of the vesicles remain unchanged. Blank vesicles were prepared by solvent exchange method by mixing 50 mL of PEG5k-P (DTC2k-TMC15k) and Mal-PEG7.5k-P (DTC2k-TMC15k) in DMSO solution (10 mg / mL) was added to 950 μL HEPES buffer solution (pH 7.4), and then dialyzed in PB (pH 7.4, 5 mM) for 8 hours, and the dialysis medium was changed 5 times. Then add 1.2-fold excess of HA-SH (17 kDa) relative to the Mal group under nitrogen, shake at 37°C overnight, and finally use an ultrafiltration tube for ultrafiltration and centrifugation (MWCO 100 kDa, ...
Embodiment 3
[0048] Example 3 Post-surface modification of HA vesicles loaded with granzyme B (HA-RCP-GrB) and reduction-triggered drug release
[0049] The loading of HA vesicles to proteins such as GrB is the same as in Example 2. Under stirring at room temperature, 50 μL of a DMSO solution (10 mg / mL) of PEG5k-P (DTC2k-TMC15k) and Mal-PEG7.5k-P (DTC2k-TMC15k) mixed in a specific ratio was added to 950 μL containing a certain GrB in HEPES buffer solution (pH 7.4, 5 mM), transfer to dialysis bag (MWCO 350 kDa) after the dropwise addition, dialyze in PB (pH 7.4, 5 mM) solution for 8 hours, and change dialysis 5 times during the period medium. The method of modifying GrB-loaded vesicles with HA-SH is the same as that of blank vesicles: add a specific amount of HA-SH (17 kDa) 1.2 times the amount of the Mal group to the resulting vesicle solution under nitrogen protection, 37 Shake overnight at ℃, and finally use an ultrafiltration tube for ultrafiltration and centrifugation (MWCO100 kDa, 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com