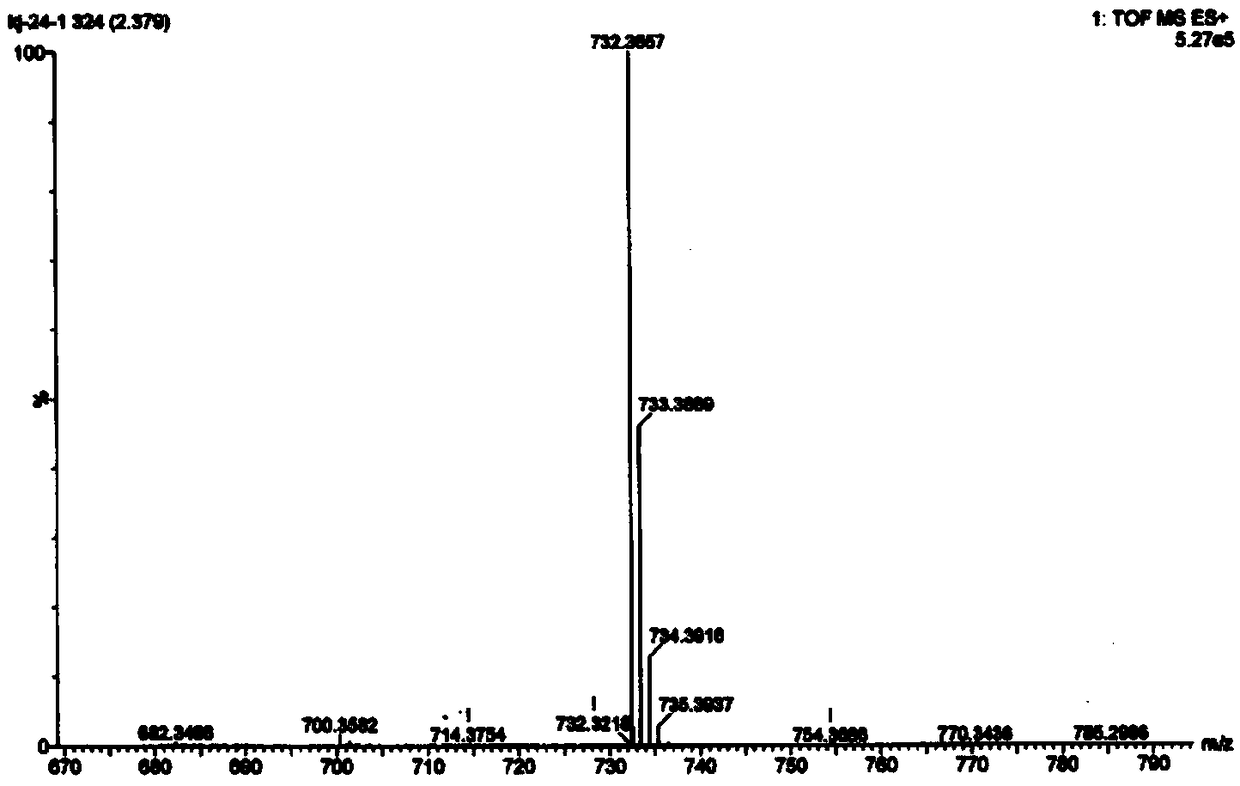
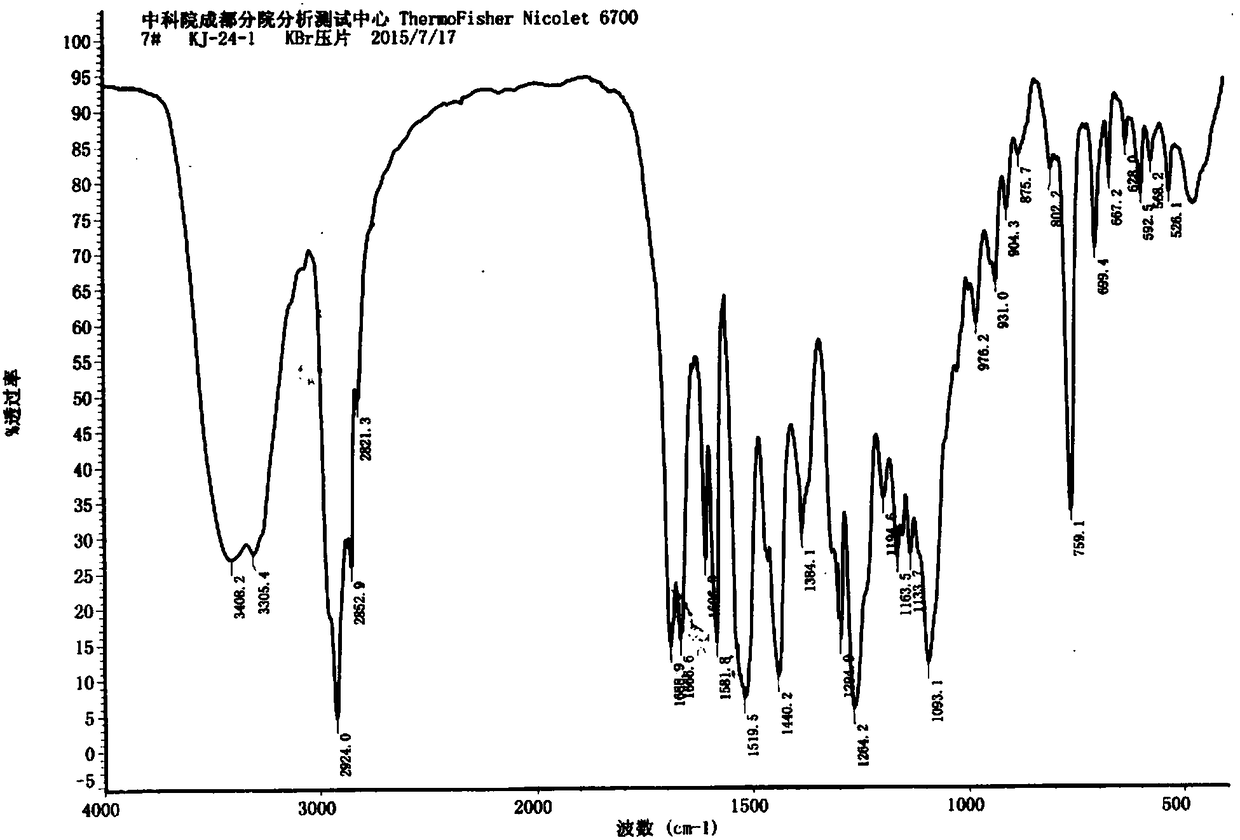
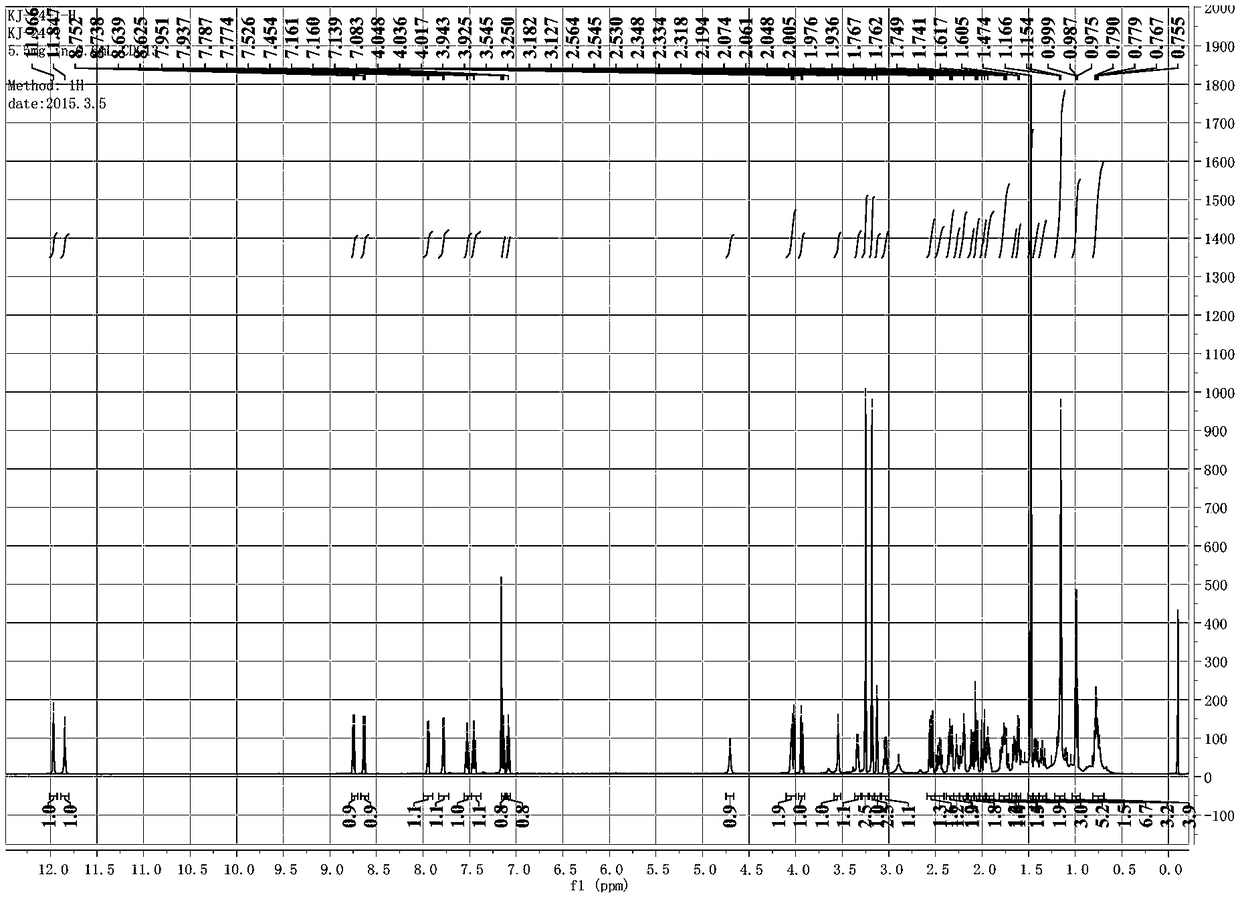
A class of C19 diterpene alkaloids with novel structure, and uses thereof
A technology of uses and drugs, applied in the field of medicine, can solve the problems of fluctuating symptoms, restoring degenerated neurons, and limited treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The preparation of embodiment 1 compound of the present invention
[0086] Take 4.3 kg of Aconitum apetalum tuber tuber of Aconitum apetalum. of Ranunculaceae, extract the dry root of Aconitum apetalum four times with 95% ethanol at room temperature, soak for 4 days each time, and concentrate under reduced pressure to obtain Extract (360g).
[0087] The extract is dissolved in water at 50°C, the pH value is adjusted to 3.0 with 10% w / w hydrochloric acid solution, and then extracted with petroleum ether and ethyl acetate successively. The aqueous solution was then adjusted to pH 9.4 with 24% w / w concentrated ammonia water, extracted with dichloromethane, and the dichloromethane fraction was concentrated under reduced pressure to obtain the total alkaloid (40 g).
[0088] Using normal phase silica gel column chromatography, CH 2 Cl 2 :CH 3 OH (1:0-0:1) for gradient elution, detected by thin-layer chromatography, combined fractions in sequence, and obtained eight parts...
experiment example 1
[0106] Experimental example 1 Inhibitory effect of compounds of the present invention on oxidative damage of SH-SY5Y cells
[0107] Take out the SH-SY5Y cell line frozen in liquid nitrogen, and quickly place it in a 37°C water bath and shake it to melt it. Take it out, move it to the ultra-clean workbench, scrub and disinfect it with 75% alcohol, take out the cell suspension under aseptic conditions, pour it into a sterile centrifuge tube, and add an appropriate amount of DMEM complete culture solution containing 10% fetal bovine serum, 1000r / min, Centrifuge for 5 minutes, suck off the supernatant, and then centrifuge once more with the above culture medium. SH-SY5Y cells were diluted with DMEM complete culture medium containing 10% fetal bovine serum to contain 1×10 per 1 mL 5 A cell suspension of 1 cell was seeded in a cell culture flask. After inoculation, place the culture flask at 37°C, 5% CO 2 Conditional CO 2 cultured in an incubator. The culture medium was replace...
experiment example 2
[0113] Experimental example 2 compounds of the present invention to MPP + Protective Effect of Induced Apoptotic SH-SY5Y Cells
[0114] SH-SY5Y cells were cultured in Basic DMEM medium containing 10% fetal bovine serum and incubated at 37°C, 5% CO 2 , in an incubator with saturated humidity, after the cells grow to a suitable density, trypsinize and passage. SH-SY5Y cells were digested and seeded in 96-well cell culture plates. After the cells adhered to the wall, MPP with a concentration of 1.8mM was added. + And the test samples Apetaldine A, Apetaldine B, Apetaldine C, Apetaldine D, and Apetaldine E dissolved by DMSO (administration concentrations are shown in Table 4), and the blank control group only added PBS as a control. Set up three parallel wells for each sample, use an automatic calibration spectrophotometer to measure the inhibitory activity of each sample by the Ehrman method, calculate the protection rate, and detect different concentrations of Apetaldine A, Ap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com