Fluorescence detection application of switching type mercaptan fluorescence labeling reagent as well as synthesis method thereof
A fluorescence detection and fluorescence labeling technology, which is applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of no solution, complex synthesis steps, high background interference rate, etc. The effects of high labeling yield and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
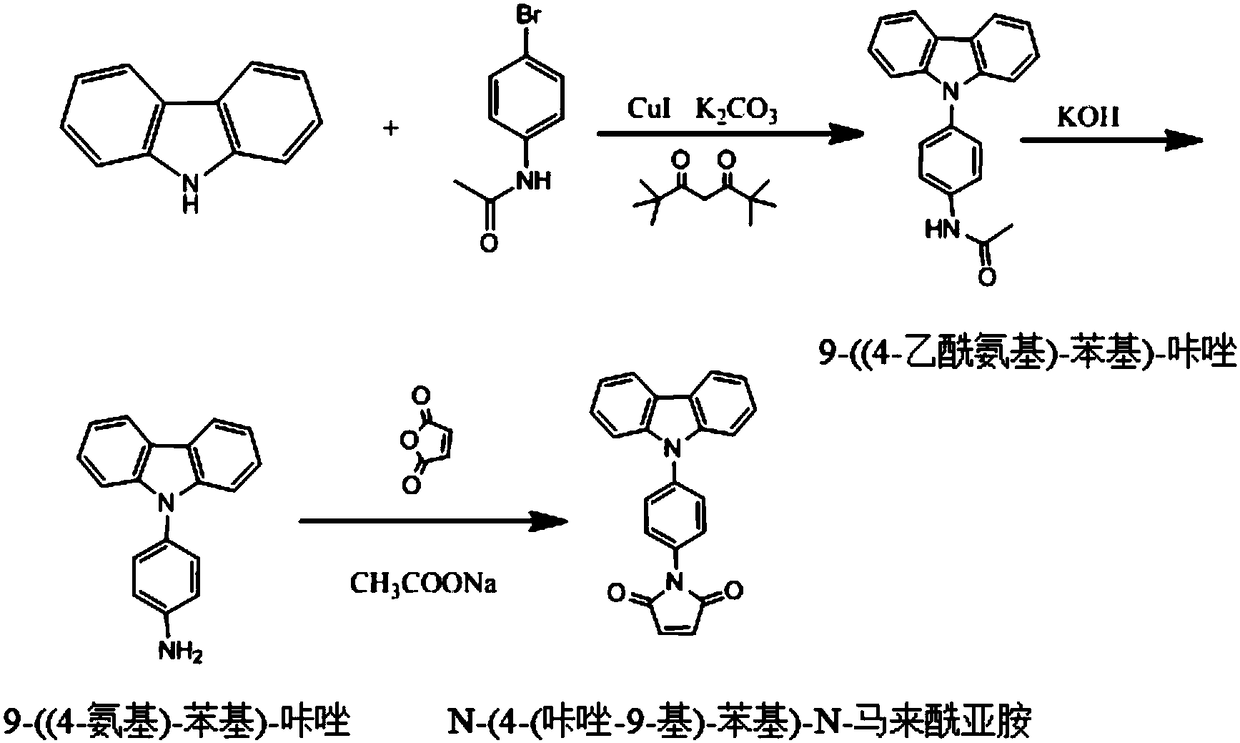
[0031] The synthesis of the switch type thiol fluorescent labeling reagent of the present invention has 3 steps.
[0032] The specific synthesis process of switch type fluorescent labeling reagent is as follows figure 1 As shown, the operation steps are as follows:
[0033] 1. Preparation of Intermediate Ⅰ 9-((4-Acetylamino)-phenyl)-carbazole
[0034] Add 10g carbazole and 16.5g 4-bromoacetanilide to a 250mL three-necked flask, add 150mL dimethyl sulfoxide as a solvent, add 5g potassium carbonate, 2.5g cuprous iodide and 3mL di-tert-valeryl methane, and react in an oil bath at 130°C for 12h Overnight; after the reaction is over, the reaction solution is cooled to room temperature and 25°C and then suction filtered, and then the suction filtrate is poured into a 25wt% NaCl aqueous solution to precipitate a precipitate. The precipitated solid is recovered and dried to obtain Intermediate I9-((4-Acetylamino)- (Phenyl)-carbazole, the yield was 85 wt%.
[0035] 2. Preparation of intermedi...
Embodiment 2
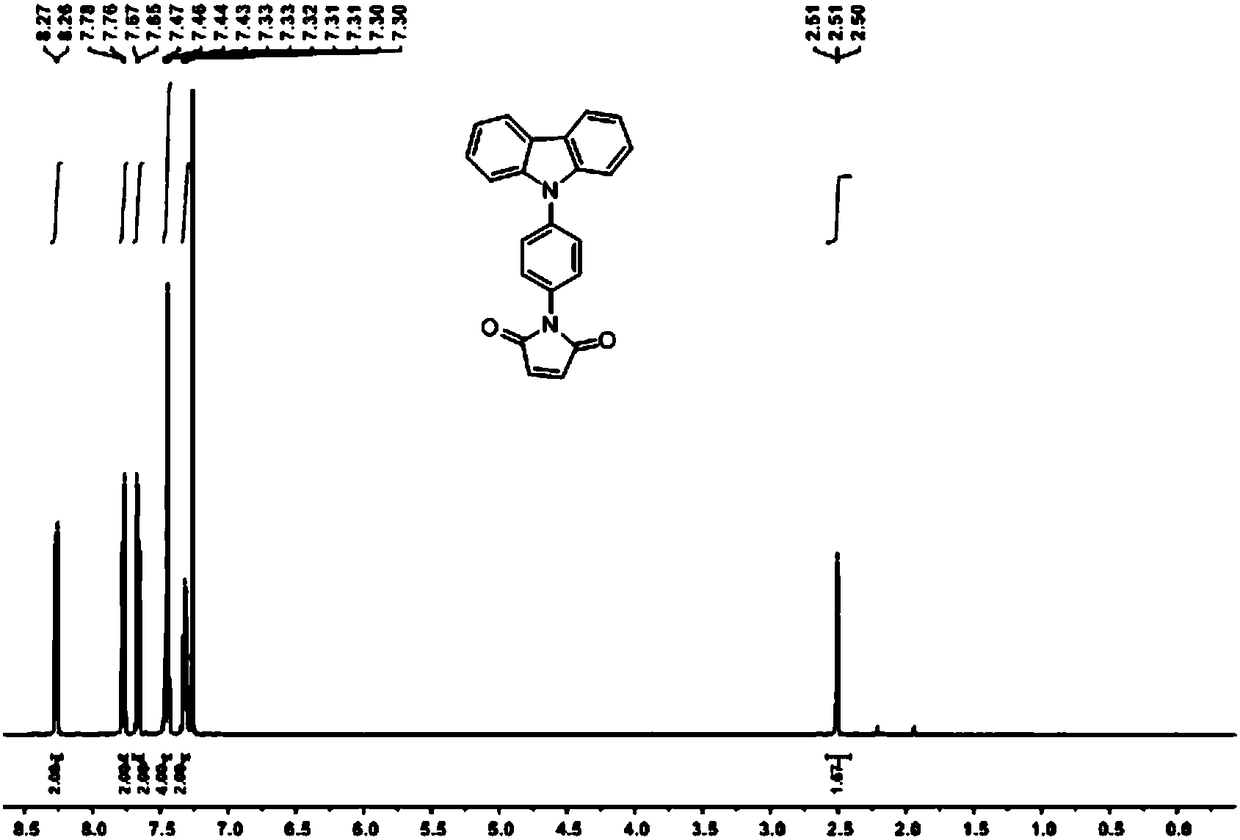
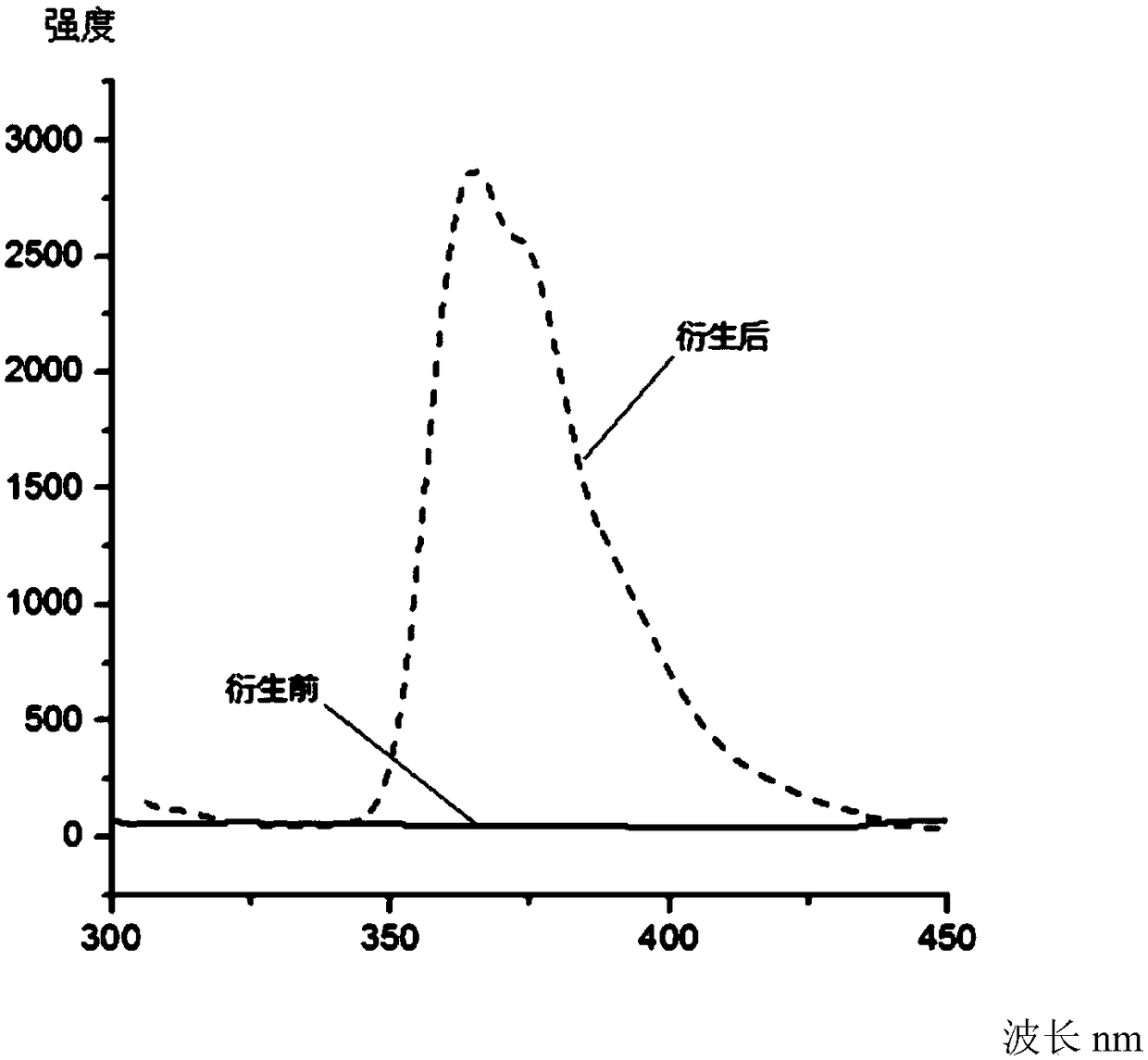
[0044] In this example, the method described in Example 1 was used to synthesize the switch type thiol fluorescent labeling reagent of the present invention, and at the same time, the fluorescent properties of the switch type fluorescent labeling reagent were verified:
[0045] A 1μM N-(4-(carbazol-9-yl)-phenyl)-N-maleimide solution and a phenylethyl mercaptan standard solution of the same concentration were prepared with acetonitrile. Such as image 3 As shown, before the labeling reagent reacts with benzene ethyl mercaptan, the reagent has no fluorescence, and the fluorescence is significantly increased after the reaction. The best excitation and emission wavelengths of fluorescence are 290nm and 368nm, respectively. At the same time, using tryptophan standard solution as the reference solution, calculate the fluorescence quantum yield of N-(4-(carbazol-9-yl)-phenyl)-N-maleimide and its thiol derivative respectively Are 0.004 and 0.415.
Embodiment 3
[0047] In this example, the method described in Example 1 was used to synthesize the switch-type thiol fluorescent labeling reagent of the present invention, and the fluorescent labeling reagent was applied to the detection of sulfhydryl samples (grilled shrimp):
[0048] (1) Prepare a phosphate buffer solution with pH=7.4 and a concentration of 10mM; prepare a concentration of 1×10 separately -4 mol / L of the acetonitrile solution of the fluorescent labeling reagent of the present invention; the selected mercaptan standard contains five kinds, namely 2-methyl-1-propanethiol, 2-methyl-1-butanethiol, and furfuryl sulfide Alcohol, 2-methyl-3-furan mercaptan and benzene ethyl mercaptan, prepare five groups of different concentrations of mixed standard acetonitrile solution, the concentration of each group is 1μM, 10μM, 100μM, 1mM and 10mM.
[0049] (2) Add 50μL of phosphate buffer solution, 100μL of mercaptan standard solution and 50μL of acetonitrile solution of fluorescent labeling re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com