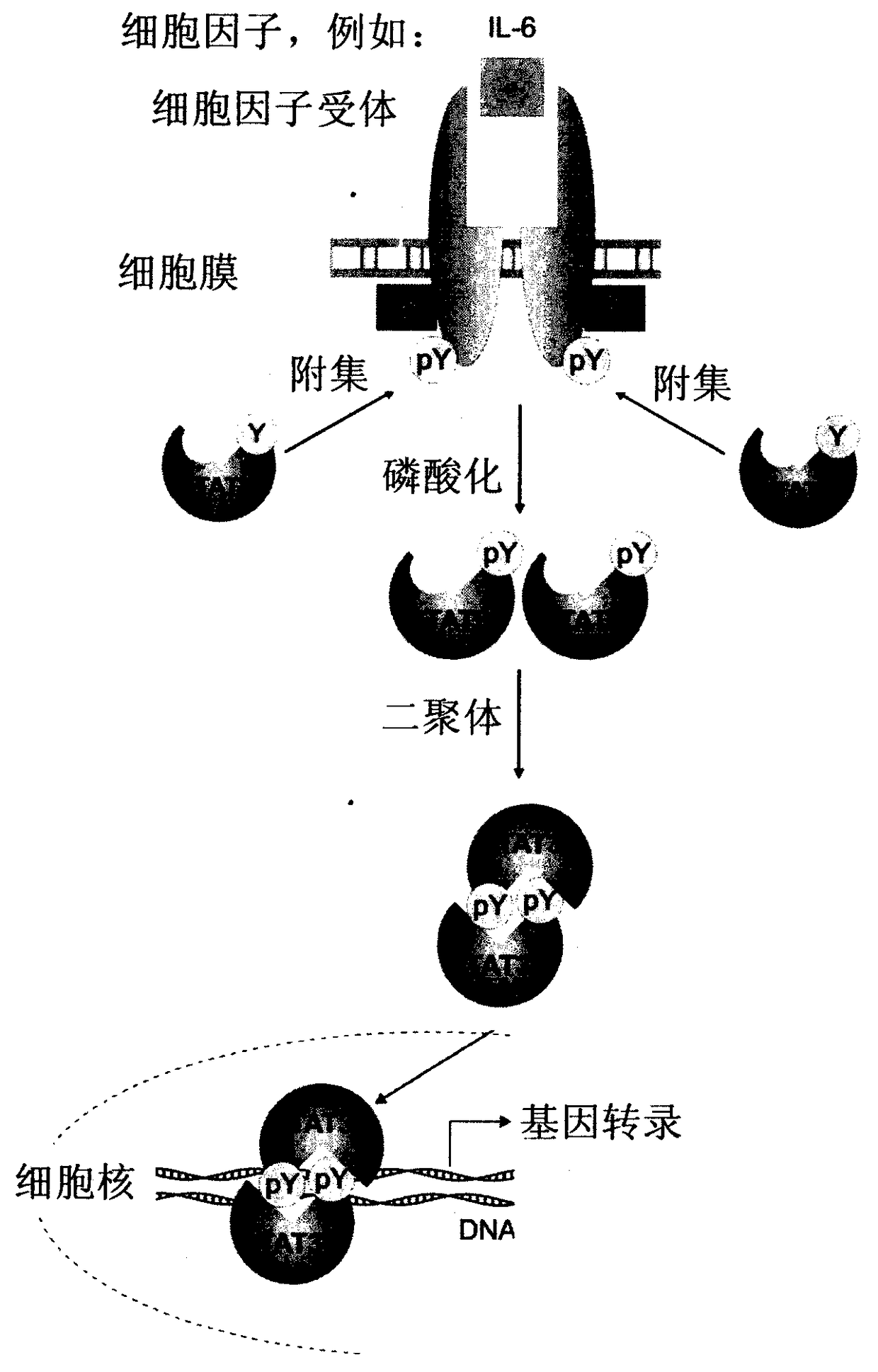
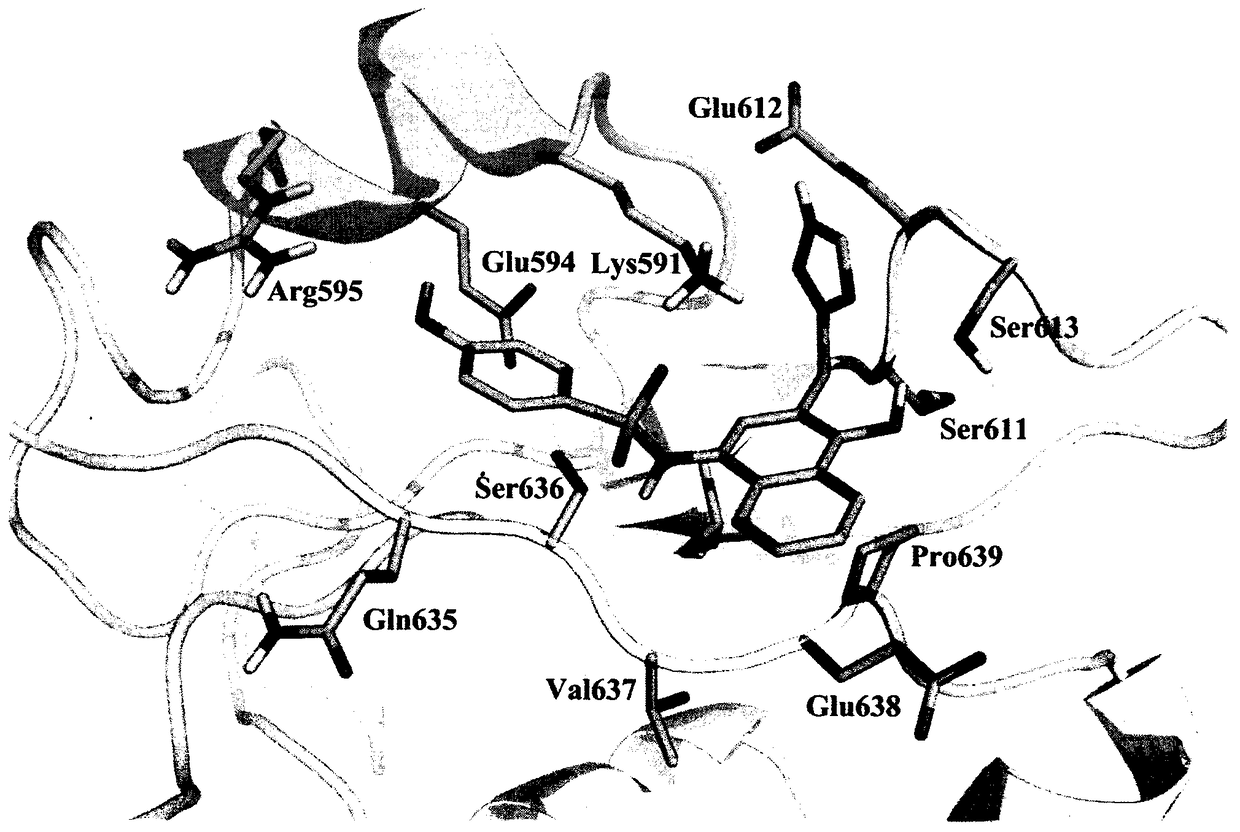
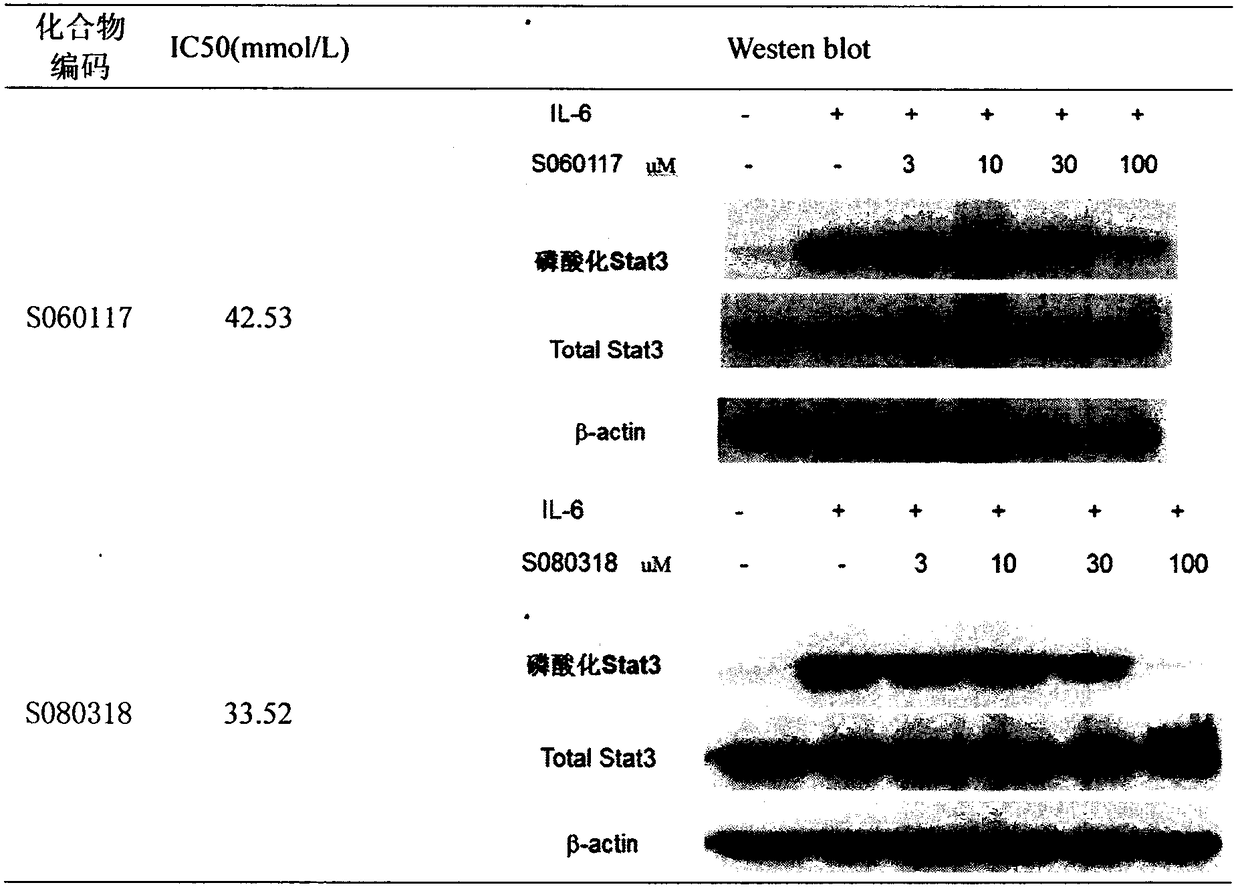
Preparation and uses of a class of novel signal transducer and activator of transcription type3 (STAT3) inhibitors
A signal transduction and inhibitor technology, applied in organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve the problems of difficult modification of natural compounds, easy to cause drug resistance, and no increase in biological activity of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: N-(3-((1H-1,2,4-triazole-5-)thio)-4-hydroxynaphthalene-1-yl)-4-bromobenzenesulfonamide (compound S060117) Synthesis
[0047]
[0048] Compound S060117
[0049] 1) Synthesis of N-(4-(4H)naphthyloneimine)-4-bromo-benzenesulfonamide:
[0050]
[0051] 4-Amino-1-naphthol hydrochloride (1.95g, 10mmol) solid was dissolved in 15ml of pyridine, 4-bromobenzenesulfonyl chloride (3.82g, 15mmol) was added, and the reaction was stirred for 5 hours; TLC detected that the reaction was complete, evaporated Pyridine was removed, the residual solid was washed with water and recrystallized from ethyl acetate to give a yellow solid as N-(4-hydroxy-1-naphthyl)-4-bromobenzenesulfonamide.
[0052]Disperse N-(4-hydroxy-1-naphthyl)-4-bromobenzenesulfonamide (3.76g, 10mmol) in 50ml of dichloromethane, add Ag 2 O (2.78g, 12mmol), stirred and reacted for 30 minutes, TLC detected that the reaction was complete, filtered, the filter cake was washed with dichloromethane, the filtr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com