Method for preparing phenylglyoxylic acid ester from mandelic acid ester by catalytic oxidation
A technology of acetophenone ester and mandelate, which is applied in the field of catalytic oxidation of mandelate to prepare acetophenone ester, which can solve the problems of low catalytic oxidant efficiency, difficult oxidation of oxidant, long reaction time, etc., and achieves cheap and efficient catalyst , few reaction steps and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
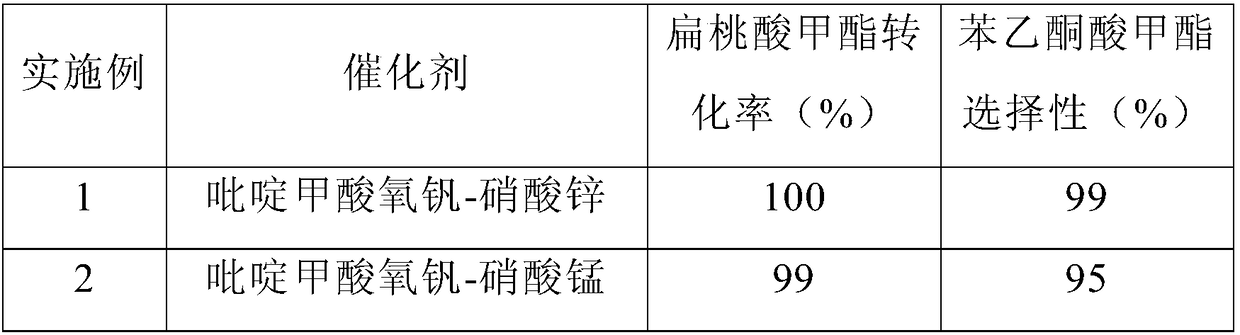
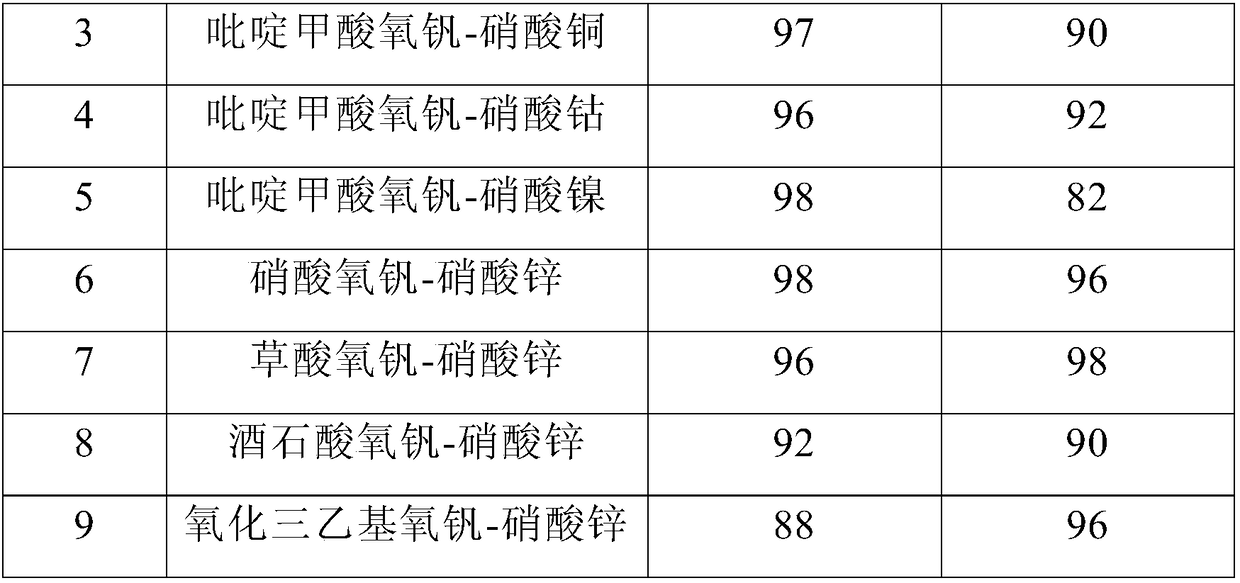
[0029] Embodiment 1-9 discloses the impact of different composite catalyst types on the conversion rate of methyl mandelic acid and the selectivity of methyl acetophenone, and the specific experimental process is described as follows:
[0030] The method for preparing acetophenone ester by catalytic oxidation of mandelic acid ester comprises the following steps: 5mmol methyl mandelate, 5mol% vanadium oxy compound, 5mol% zinc nitrate, 5ml acetonitrile is added in the 25ml reactor, after the airtight reactor, to the reactor filled with 0.2MPaO 2 , heated to 80°C under stirring, and kept for 4h, cooled to room temperature after the reaction was completed, and the product was analyzed quantitatively by gas chromatography, and the analysis results are shown in Table 1:
[0031] Table 1: Effects of different catalyst types on the conversion rate of methyl mandelate and the selectivity of methyl acetophenone
[0032]
[0033]
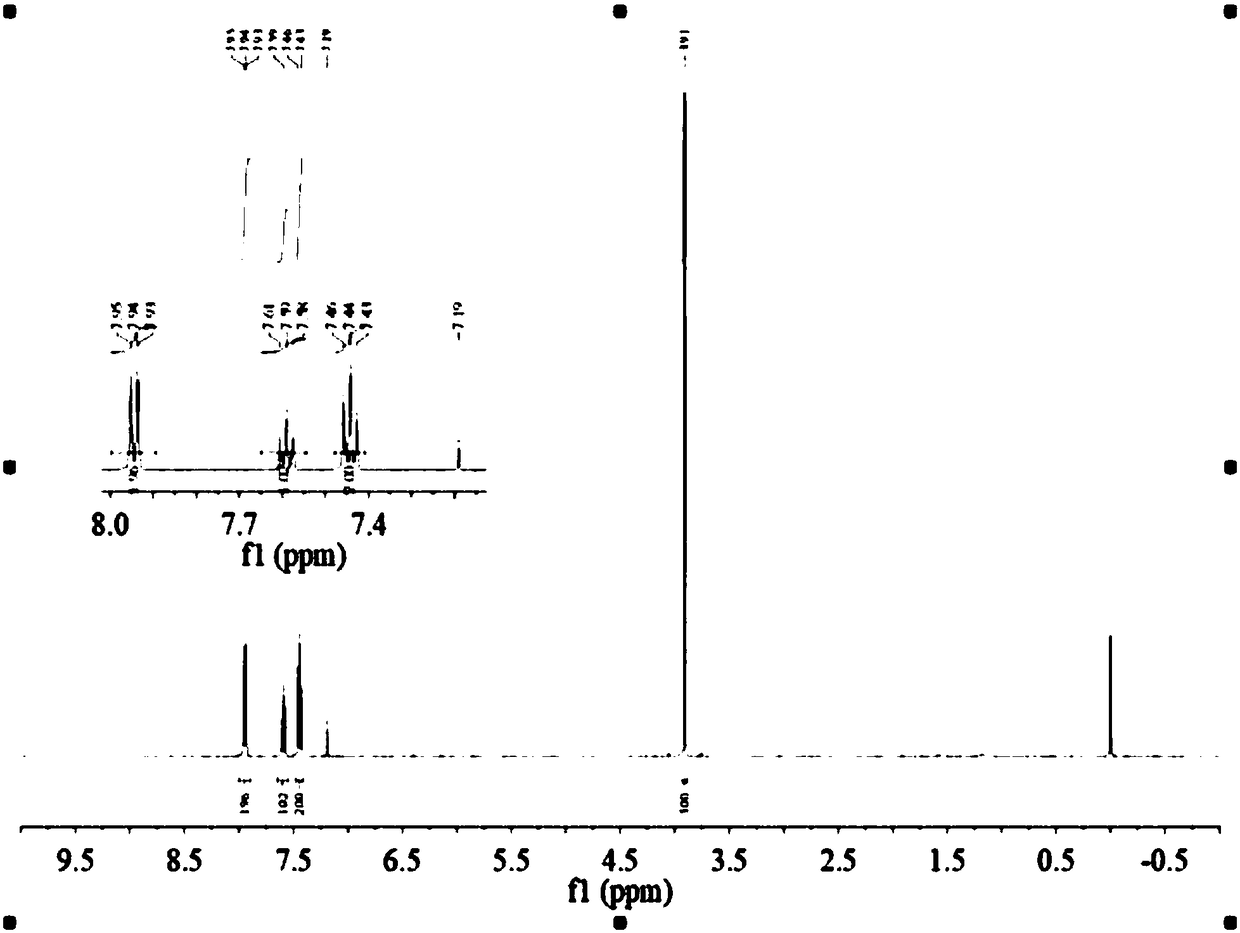
[0034] figure 1 For the purification product met...
Embodiment 10-14
[0036] Embodiment 10-14 discloses the impact of compounding the amount of addition of catalyst on the conversion rate of methyl mandelate and the selectivity of methyl acetophenone, and the specific experimental process is described as follows:
[0037] The method for preparing acetophenone ester by catalytic oxidation of mandelic acid ester comprises the following steps: a certain amount of catalyzer (vanadyl picolinate / zinc nitrate=100%), 5mmol methyl mandelic acid, 5ml acetonitrile is added in the 25ml reactor, to Fill the reactor with 0.2MPaO 2 , heated to 80°C under stirring, and kept for 4h, cooled to room temperature after the reaction was completed, and the product was analyzed quantitatively by gas chromatography, and the analysis results are shown in Table 2:
[0038] Table 2: Effect of catalyst addition on the conversion rate of methyl mandelate and the selectivity of methyl acetophenone
[0039]
Embodiment 15-18
[0041]Embodiment 15-18 discloses the influence of the molar ratio of transition metal nitrate and vanadium oxy compound on the conversion rate of methyl mandelate and the selectivity of methyl acetophenone, and the specific experimental process is described as follows:
[0042] The method for preparing acetophenone ester by catalytic oxidation of mandelic acid ester comprises the following steps: 5mmol methyl mandelate, 5mol% vanadyl picolinate, a certain amount of zinc nitrate, 5ml acetonitrile is added in a 25ml reactor, and the reactor is filled with 0.2MPaO 2 , heated to 80°C under stirring, and maintained for 4h, cooled to room temperature after the reaction was completed, and the product was analyzed quantitatively by gas chromatography, and the analysis results are shown in Table 3:
[0043] Table 3: The effect of the molar ratio of transition metal nitrate to vanadyl compound on the conversion of methyl mandelate and the selectivity of methyl acetophenone
[0044] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com