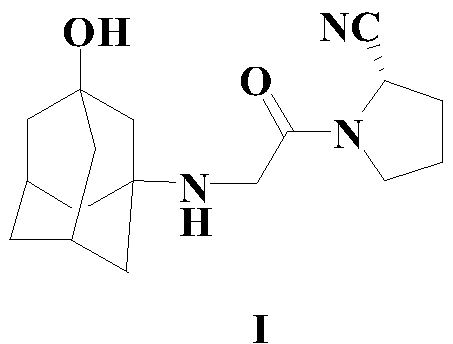
A kind of synthetic method of vildagliptin
A synthesis method and compound technology, applied in the field of pharmaceutical synthesis, can solve problems such as unfavorable environmental protection, increase product cost, high price, etc., and achieve the effects of less by-products, low cost, and improved reaction efficiency and conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of Compound II
[0037] Glyoxylic acid monohydrate 27.6g (0.30mol, 1.5eq), 3-amino-1-adamantanol 33.5g (0.20mol, 1.0eq), diisopropylethylamine 38.8g (0.30mol, 1.5eq ) and 250g of tetrahydrofuran were put into the reaction flask, 51.1g (0.50mol, 2.5eq) of acetic anhydride was added dropwise, heated to reflux and stirred for 2 hours, the remaining raw materials were controlled in HPLC to be less than 1%, 50g of water was added, stirred for 30 minutes, and separated, The aqueous layer was extracted once with 50 g of dichloromethane, and the organic layer was concentrated under reduced pressure to obtain 42.8 g of off-white solid Compound II with a yield of 96.1% and a purity of 96.6%. 1H-NMR (400MHz, DMSO-d6): 4.34 (s, 1H), 2.08 (m, 2H), 1.46-1.24 (m, 12H), 7.73 (s, 1H), 13.24 (s, 1H); m / z(ESI + )224.0(MH) + .
Embodiment 2
[0039] Synthesis of Compound II
[0040] Glyoxylic acid monohydrate 22.1g (0.24mol, 1.2eq), 3-amino-1-adamantanol 33.5g (0.20mol, 1.0eq), triethylamine 20.2g (0.20mol, 1.0eq) and 250g di Chloromethane was put into the reaction flask, 40.9g (0.40mol, 2.0eq) of acetic anhydride was added dropwise, stirred at room temperature for 10 hours, and the remaining raw materials were controlled by HPLC to less than 3%, 50g of water was added, stirred for 30 minutes, separated, and the water layer was used 50g of dichloromethane was extracted once, and the organic layer was concentrated under reduced pressure to obtain 41.8g of off-white solid compound II, with a yield of 93.7% and a purity of 94.2%; m / z (ESI + )224.0(MH) + .
Embodiment 3
[0042] Synthesis of Compound III
[0043]Under nitrogen protection, compound II 42.8g (0.19mol, 1.0eq), (S)-pyrrolidine-2-carbonitrile 18.3g (0.19mol, 1.0eq), boric acid tris(2,2, Put 11.7g (38mmol, 0.2eq) of 2-trifluoroethyl) ester and 350g toluene into the reaction flask connected with the water separator, heat and reflux for 12 hours, separate 3.4g of water, crystallize at room temperature, filter, filter 50.5g of white solid compound III was obtained, with a yield of 88.2% and a purity of 98.6%. 1 H-NMR(400MHz, CDCl3):1.45-1.64(m,14H),2.09(m,1H),2.25-2.38(m,4H),3.40-3.54(m,1H),3.57-3.67(m,1H ),4.70-4.75(t,1H),7.79(s,1H).m / z(ESI + )302.2 (MH) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com