Method for producing hydrogen peroxide by electrocatalytic reduction of oxygen
An electrocatalysis, hydrogen peroxide technology, applied in the field of electrochemistry, can solve the problems of high cost, high energy consumption, dangerous transportation, etc., and achieve the effects of high selectivity, large specific surface area and fast diffusion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
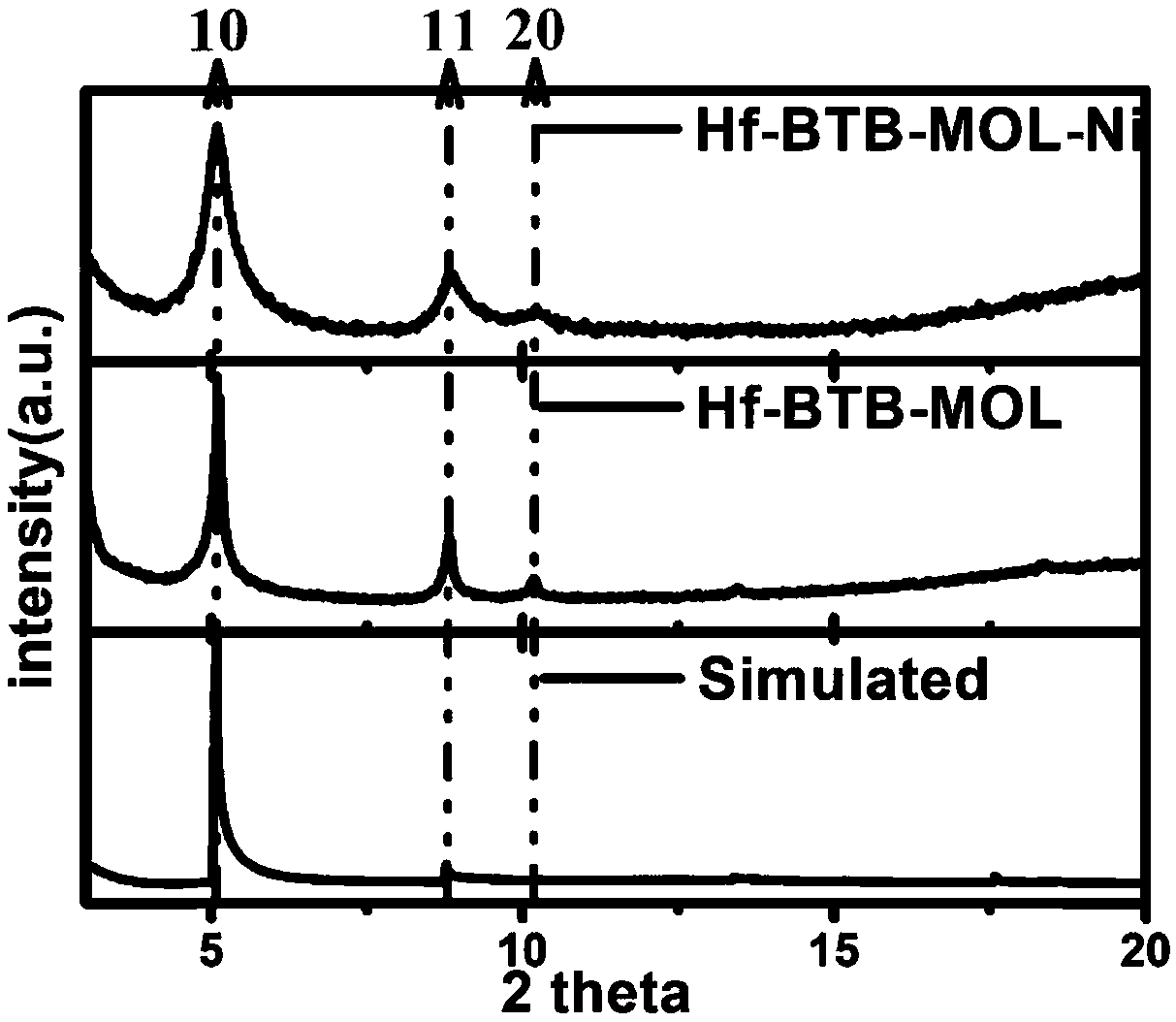
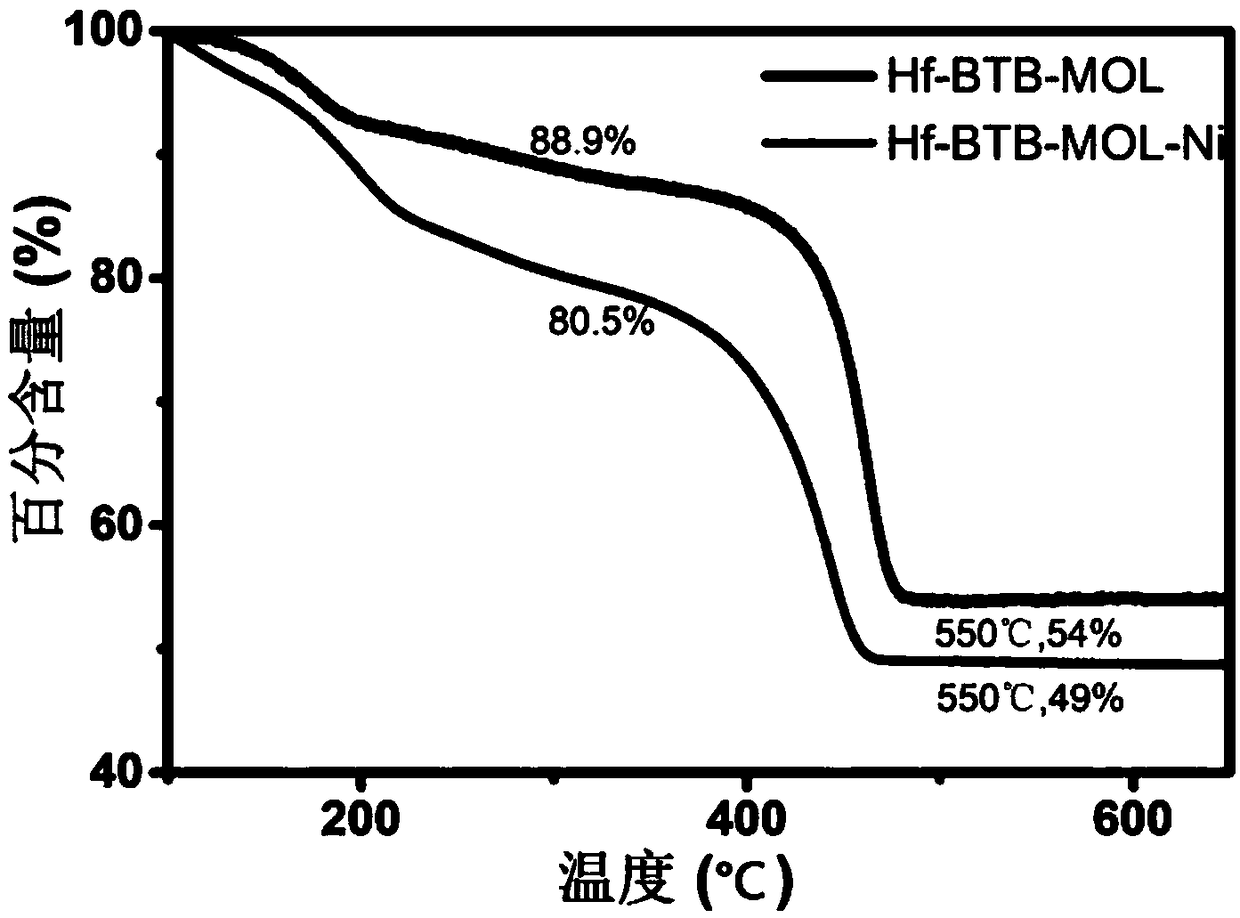
[0036] In this example, the US PINE rotating disc electrode and H-type electrolytic cell were used to conduct electrocatalytic tests. This embodiment uses a kind of organic molecule 1,3,5-three (4-carboxyphenyl) benzene (benzene-1,3,5-tribenzoate) as a ligand (hereinafter referred to as: H 3 BTB), based on the hexanuclear metal hafnium cluster (hereinafter referred to as: Hf 6 ) as a metal node, a metal organic layer (Metal Organic Layers-MOL) compound (hereinafter referred to as: Hf-BTB-MOL) was synthesized. Hf-BTB-MOL has a 3,6-connected two-dimensional kgd topology network structure. Hf-BTB-MOL is a network-like film that can be used to modify the electrode surface. f 6 μ in SBU 3 The protons of -OH can dissociate at neutral pH, where MOL is negatively charged. [Ni II (H 2 O) 6 ] 2+ The complex can be adsorbed on the MOL on the electrode surface by Coulomb attraction and reduce O by hydrogen bonding 2 . Ni II The dynamic site segregation leads to an efficient an...
Embodiment 2
[0057] In this example, the American PINE rotating disk electrode pair Hf-TPY-MOL-Fe was used in 0.5M KNO 3 Electrocatalytic tests were carried out in the electrolyte. This embodiment uses a kind of organic molecule 4-benzoic acid-2,2,2-terpyridine-5,5-dicarboxylic acid as a ligand (hereinafter referred to as: H 3 TPY), based on the hexanuclear metal hafnium cluster (hereinafter referred to as: Hf 6 ) as a metal node, a metal organic layer (Metal Organic Layers-MOL) compound (hereinafter referred to as: Hf-TPY-MOL) was synthesized. Hf-TPY-MOL has a 3,6-connected two-dimensional kgd topology network structure. Hf-TPY-MOL is a network-like film that can be used to modify the electrode surface. f 6 μ in SBU 3 The protons of -OH can dissociate at neutral pH, where MOL is negatively charged. Hf-TPY-MOL is similar in structure to Hf-BTB-MOL in Example 1. Hf-TPY-MOL can adsorb Fe 3+ ions for electrocatalytic oxygen reduction to produce hydrogen peroxide. At the same time, th...
Embodiment 3
[0066] In this example, the US PINE rotating disk electrode is used to pair the Zr-HHTP MOF material at 0.5M KNO 3 Electrocatalytic tests were carried out in the electrolyte. In this embodiment, 2,3,6,7,10,11-hexahydroxytriphenylene hydrate (hereinafter referred to as: HHTP) is used as ligand and ZrOCl 2 metal ions, using the bottom-up approach to synthesize ultrathin Zr-HHTP MOF materials using LB technology, which can have a good catalytic effect in oxygen reduction electrocatalysis and produce H with a selectivity of up to 80%. 2 o 2 .
[0067] 1. Pre-experiment preparation:
[0068] First, add 300mL H 2 Add 300uL 0.01mol / L metal ion solution to O, and this solution is the subphase solution. Second, wash the sink part and sliding barrier of the KSV membrane analyzer PTFE in one direction with ethanol, and then wash with deionized water. Add the prepared subphase solution into the tank until the liquid level is about 1-2mm above the tank level. Soak the Wilhelmy piece...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com