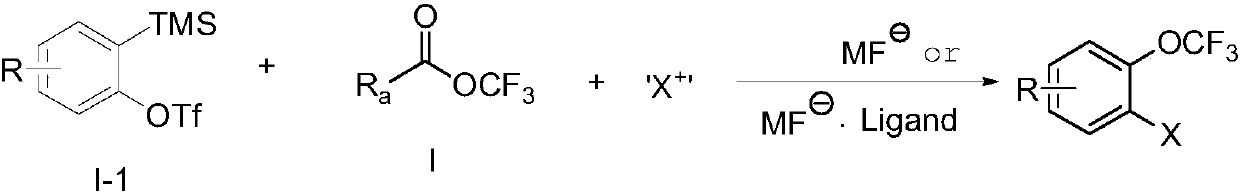Perfluoro alkoxylation reagent and preparation method and application thereof
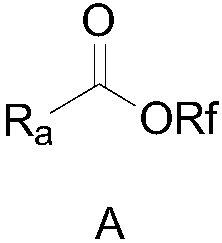
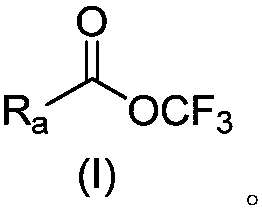
A perfluoroalkoxy and alkyl technology is used in the preparation of trifluoromethoxy ester compounds. The trifluoromethoxyl group is introduced into the field of organic molecules by trifluoromethoxyl group, which can solve the problem of trifluoromethoxyl group. Difficulty in oxygen groups, scarcity of trifluoromethoxylation reagents, inconvenient operation and use, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143]
[0144] KF (65.4g, 1125mmol) and 18-crown-6 (8.9g, 34mmol) were added to the 350mL sealed tube I equipped with a strong stirring bar baked by a heat gun, and in the glove box. The glove box was removed, 230 mL of acetonitrile was added under nitrogen protection, and triphosgene (37.1 g, 125 mmol) was added under cooling in a dry ice / acetone bath, the temperature was raised to room temperature, and the reaction was carried out at room temperature for 1 hour. In another 350mL sealed tube II with a strong stirrer that has been baked by a heat gun, add KF (2.2g, 38mmol), 18-crown-6 (9.9g, 38mmol) and 100mL dry THF in the glove box, remove glove box. Pump the sealing tube II for 1-2 seconds to negative pressure, and then seal the mouth. Connect the sealed tube I and the sealed tube II through a catheter. At this time, the sealed tube I is heated to 80°C, and the sealed tube II is cooled to 78°C in a dry ice / acetone bath, and then the sealed tube is opened to transfer fluo...
Embodiment 2
[0147]
[0148] KF (65.4g, 1125mmol) and 18-crown-6 (9.3g, 35mmol) were added to the 350mL sealed tube I equipped with a strong stirring bar baked by a heat gun, and in the glove box. The glove box was removed, 230 mL of acetonitrile was added under nitrogen protection, and triphosgene (37.1 g, 125 mmol) was added under cooling in a dry ice / acetone bath, the temperature was raised to room temperature, and the reaction was carried out at room temperature for 1 hour. In another 350mL sealed tube II equipped with a strong stirring bar baked by a heat gun, add TBAT (40.5g, 75mmol) and 100mL dry tetrahydrofuran into the glove box, and remove it from the glove box. Pump the sealing tube II for 1-2 seconds to negative pressure, and then seal the mouth. Connect the sealed tube I and the sealed tube II through a catheter. At this time, the sealed tube I is heated to 80°C, and the sealed tube II is cooled to 78°C in a dry ice / acetone bath, and then the sealed tube is opened to transf...
Embodiment 3
[0151]
[0152] Add NaF (1.26g, 30mmol) to the 10mL sealed tube I equipped with a strong stirrer that has been baked by a heat gun, and in the glove box. Remove the glove box, add Ishikawa's Reagent (6.69g, 30mmol) under nitrogen protection, add trifluoroacetic acid (1.71g, 15mmol) under cooling in a dry ice / acetone bath, warm to room temperature, and react at room temperature for 2 hours. Add TBAT (2.43g, 4.5mmol) and 6mL dry tetrahydrofuran to another 10mL sealed tube II equipped with a strong stirrer that has been baked by a heat gun, and remove it from the glove box. Pump the sealing tube II for 1-2 seconds to negative pressure, and then seal the mouth. Connect the sealed tube I and the sealed tube II through a catheter. At this time, the sealed tube I is heated to 50°C, and the sealed tube II is cooled to 78°C in a dry ice / acetone bath, then the sealed tube is opened, and the trifluoroacetyl fluoride is transferred from the sealed tube I to In Lock II, it took 2 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com