M-hydroxy acetophenone preparation method
A technology of m-hydroxyacetophenone and hydroxyacetophenone, which is applied in the field of preparation of m-hydroxyacetophenone, can solve the problems of large waste water discharge, high environmental protection cost, and high cost, and achieve small waste water discharge, avoid pollution, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
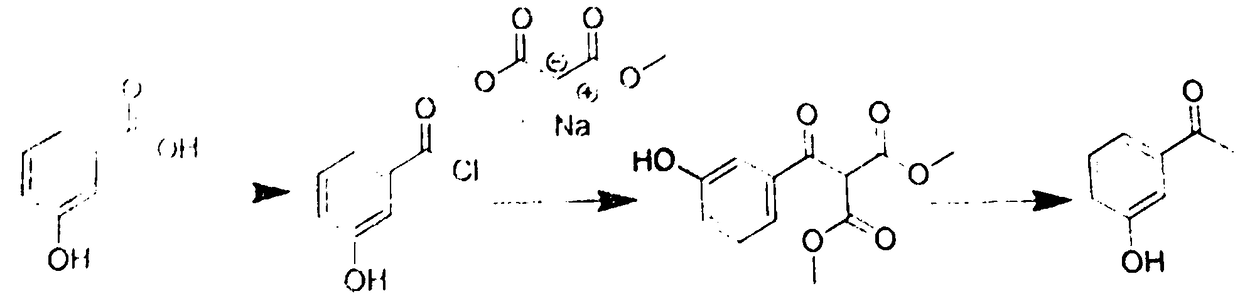
[0031] A preparation method of m-hydroxyacetophenone, which comprises the following steps:
[0032] (1) Chlorination, dissolve meta-hydroxybenzoic acid in toluene, add 4-dimethylaminopyridine as a catalyst, and then add thionyl chloride for chlorination to obtain meta-hydroxybenzoic acid chloride, which is then concentrated and evaporated. Remove thionyl chloride to obtain m-hydroxybenzoic acid chloride-toluene solution;
[0033] (2) Dissolve dimethyl malonate sodium salt in toluene to obtain dimethyl malonate sodium salt-toluene solution, and then add the m-hydroxybenzoic acid chloride-toluene solution obtained in step (1) dropwise to the malonate Dimethyl acid sodium salt-toluene solution, and then react, and reflux reaction is performed at a reaction temperature of 60°C for 4 hours to obtain product A;
[0034] (3) Add the product A obtained in step (2) to a hydrochloric acid solution, and perform a hydrolysis reaction to obtain m-hydroxyacetophenone.
[0035] In the step (1) the ...
Embodiment 2
[0040] A preparation method of m-hydroxyacetophenone, which comprises the following steps:
[0041] (1) Chlorination, dissolve meta-hydroxybenzoic acid in toluene, add 4-dimethylaminopyridine as a catalyst, and then add thionyl chloride for chlorination to obtain meta-hydroxybenzoic acid chloride, which is then concentrated and evaporated. Remove thionyl chloride to obtain m-hydroxybenzoic acid chloride-toluene solution;
[0042] (2) Dissolve dimethyl malonate sodium salt in toluene to obtain dimethyl malonate sodium salt-toluene solution, and then add the m-hydroxybenzoic acid chloride-toluene solution obtained in step (1) dropwise to the malonate In the dimethyl acid sodium salt-toluene solution, the reaction is carried out, and the reflux reaction is carried out at a reaction temperature of 70° C. for 4 hours to obtain product A;
[0043] (3) Add the product A obtained in step (2) to a hydrochloric acid solution, and perform a hydrolysis reaction to obtain m-hydroxyacetophenone. ...
Embodiment 3
[0049] A preparation method of m-hydroxyacetophenone, which comprises the following steps:
[0050] (1) Chlorination, dissolve meta-hydroxybenzoic acid in toluene, add 4-dimethylaminopyridine as a catalyst, and then add thionyl chloride for chlorination to obtain meta-hydroxybenzoic acid chloride, which is then concentrated and evaporated. Remove thionyl chloride to obtain m-hydroxybenzoic acid chloride-toluene solution;
[0051] (2) Dissolve dimethyl malonate sodium salt in toluene to obtain dimethyl malonate sodium salt-toluene solution, and then add the m-hydroxybenzoic acid chloride-toluene solution obtained in step (1) dropwise to the malonate In the dimethyl acid sodium salt-toluene solution, the reaction is carried out, and the reflux reaction is carried out at a reaction temperature of 75°C for 4.2 hours to obtain product A;
[0052] (3) Add the product A obtained in step (2) to a hydrochloric acid solution, and perform a hydrolysis reaction to obtain m-hydroxyacetophenone. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com