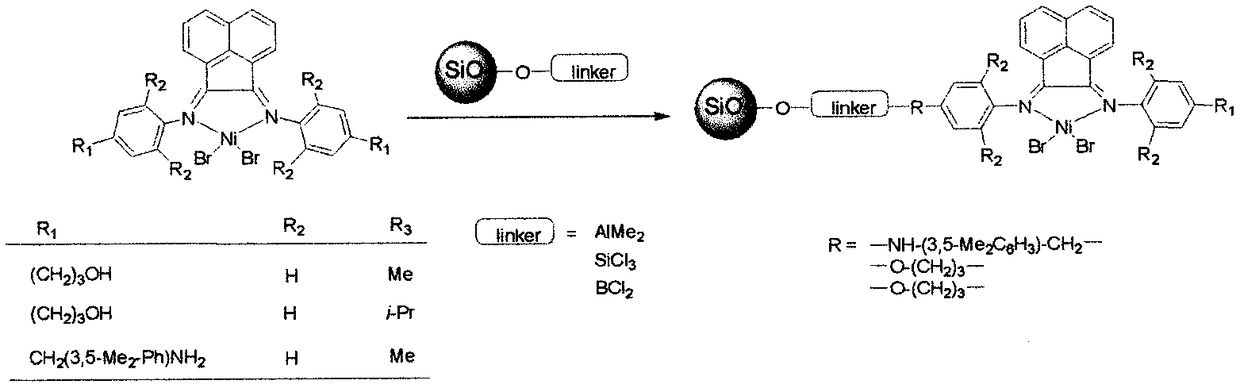
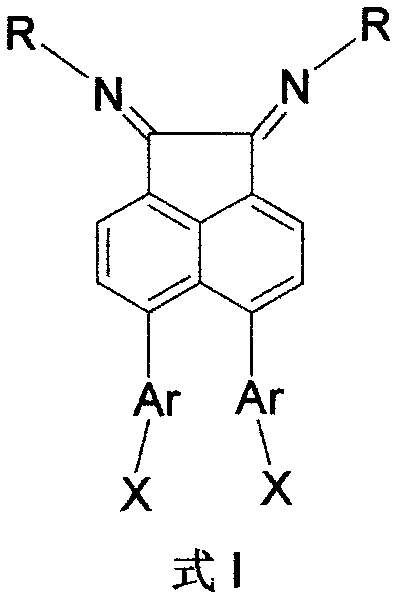
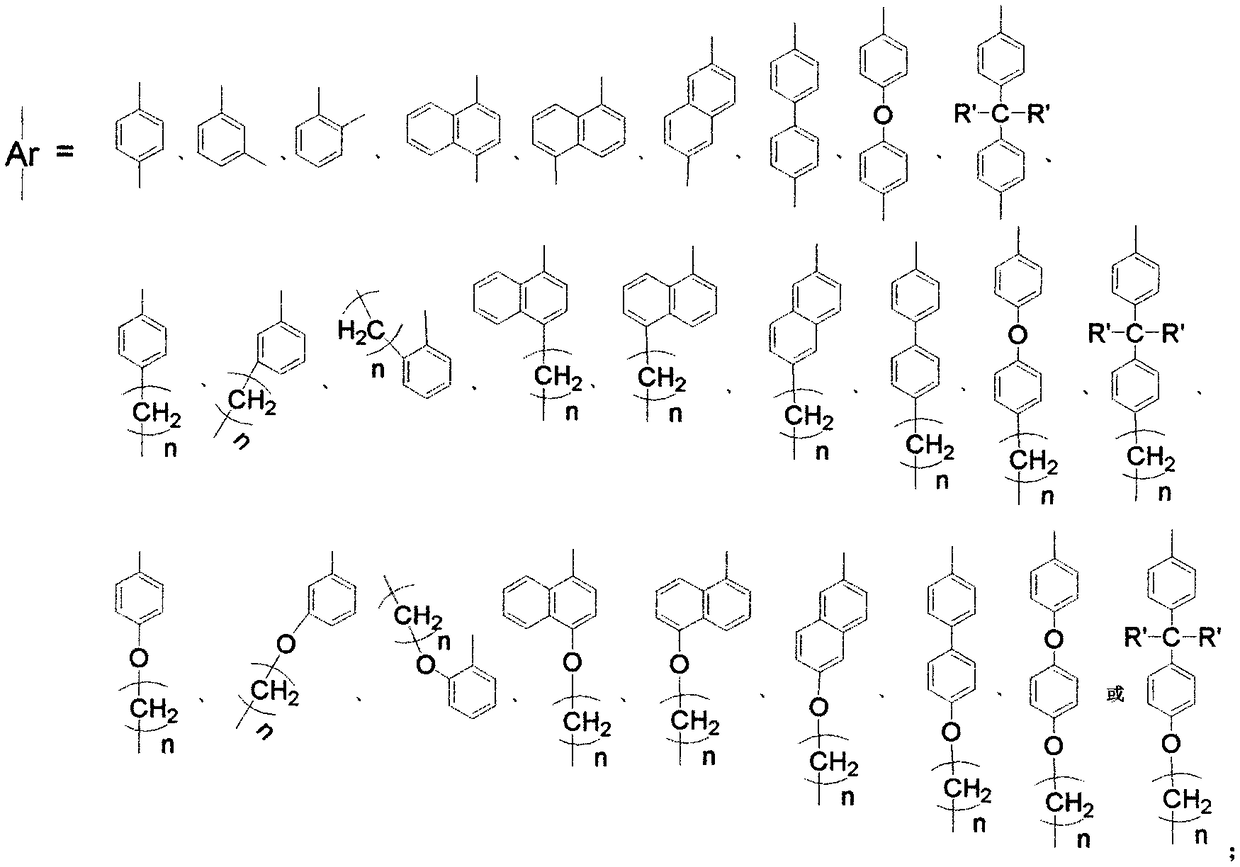
Alpha-diimine and metal application of supporting substance of metal complex thereof in olefin polymerization
A technology of metal complexes and diimine compounds, applied in the field of olefin catalysis, can solve the problems of large amount of co-catalyst, difficult to control polymer form, poor thermal stability, etc., and achieves good particle shape, reduced impact, and good thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 5,6-bis(4-hydroxymethylphenyl)acenaphthenequinone bis(2,6-diisopropyl)phenylimine b1 (that is, the structure of compound B, wherein Ar=p-methylphenyl, R= Synthesis of (2,6-diisopropyl)phenyl, X=hydroxyl):
[0041] Its preparation route is as follows:
[0042]
[0043] Synthesis of 5,6-dibromoacenaphthoquinone bis(2,6-diisopropyl)phenylimine a1:
[0044] Add 5,6-dibromoacenaphthenequinone (2.72g, 8mmol), 2,6-diisopropylaniline (3.36g, 19mmol) and 150mL of anhydrous methanol to a 250mL reaction flask, and add 10 drops With anhydrous formic acid, the mixture was refluxed for 48 hours. The reaction was tracked by chromatography until the reaction of the raw materials was complete. After the reaction mixture was cooled, a brown-red solid was obtained by suction filtration. The solid was purified by silica gel chromatography to obtain 4.69 g of a yellow solid a1 with a yield of 89%. 1 H NMR (400MHz, CDCl 3 ): δ7.79(d, J=7.8Hz, 2H), 7.32-7.29(m, 6H), 6.45(d, J=7.8Hz, 2H...
Embodiment 2
[0048] 5,6-two (4-hydroxyl phenyl) acenaphthene quinone bis (2,6-diisopropyl) phenylimine b2 (i.e. the structure of compound B, wherein Ar = p-phenyl, R = (2, Synthesis of 6-diisopropyl)phenyl, X=hydroxyl):
[0049] Its preparation route is as follows:
[0050]
[0051] Compound a1 (2.63g, 4mmol), 4-hydroxyphenylboronic acid (1.24g, 9mmol), tetrakis(triphenylphosphine) palladium (0.46g, 0.4mmol), anhydrous potassium carbonate (3.73g, 27mmol), 100mL Add toluene and 50mL water into the two-necked flask, and replace the atmosphere in the reaction flask with N 2 , heated to reflux for 10 h, cooled to room temperature, extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, filtered and suspended to dryness, and finally separated and purified by column chromatography to obtain 2.6 g of yellow solid b2 with a yield of 95%. 1 H NMR (400MHz, CDCl 3 ): δ7.32-7.29(m, 8H), 6.78-6.75(m, 6H), 6.46(d, J=8.3Hz, 4H), 3.15-3.04(m, 4H), 1.29(d, J=6.8 Hz, 12...
Embodiment 3
[0053] 5,6-two (4-aminophenyl) acenaphthene quinone bis (2,6-diisopropyl) phenylimine b3 (that is, the structure of compound B, wherein Ar = p-phenyl, R = (2, Synthesis of 6-diisopropyl)phenyl, X=amino):
[0054] Its preparation route is as follows:
[0055]
[0056] Compound a1 (2.63g, 4mmol), 4-aminophenylboronic acid (1.23g, 9mmol), tetrakis(triphenylphosphine) palladium (0.46g, 0.4mmol), anhydrous potassium carbonate (3.73g, 27mmol), 100mL Add tetrahydrofuran and 50mL water into the two-necked flask, and replace the atmosphere in the reaction flask with N 2 , heated to reflux for 10h, cooled to room temperature, extracted with dichloromethane, dried the organic phase with anhydrous sodium sulfate, filtered and spin-dried, and finally separated and purified by column chromatography to obtain 2.54g of yellow solid b3, yield 93% . 1 HNMR (400MHz, CDCl 3 ): δ7.29-7.26(m, 8H), 6.70(d, J=8.2Hz, 6H), 6.29(d, J=8.2Hz, 4H), 3.16-3.02(m, 4H), 1.26(d, J=6.7Hz, 12H), 1.03 (d, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Catalytic activity | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com