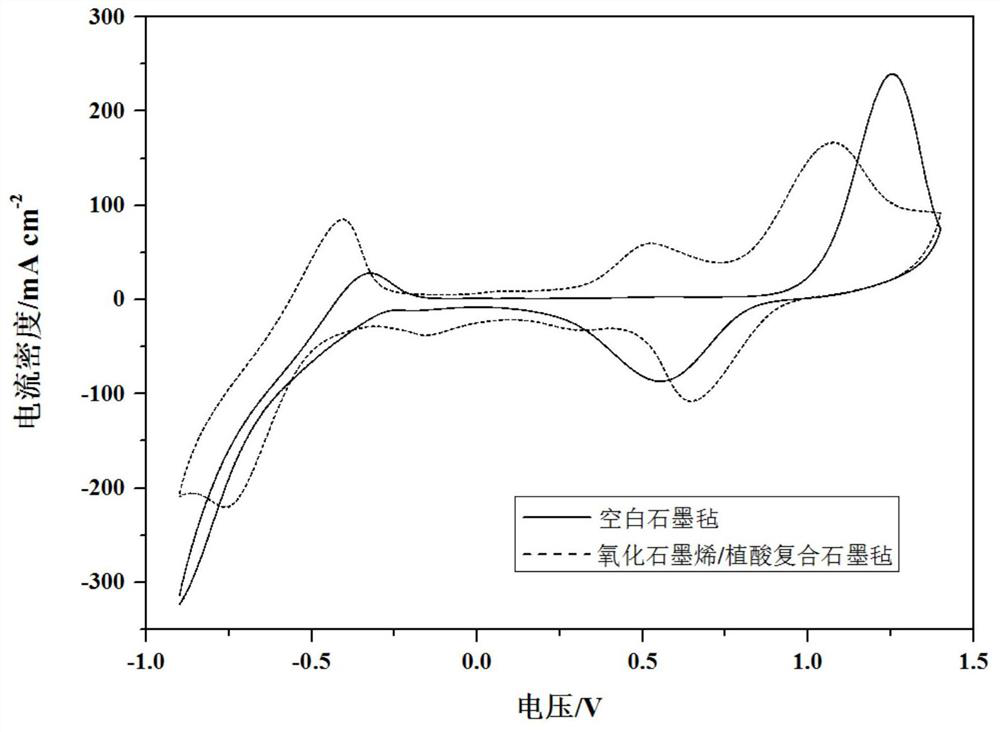
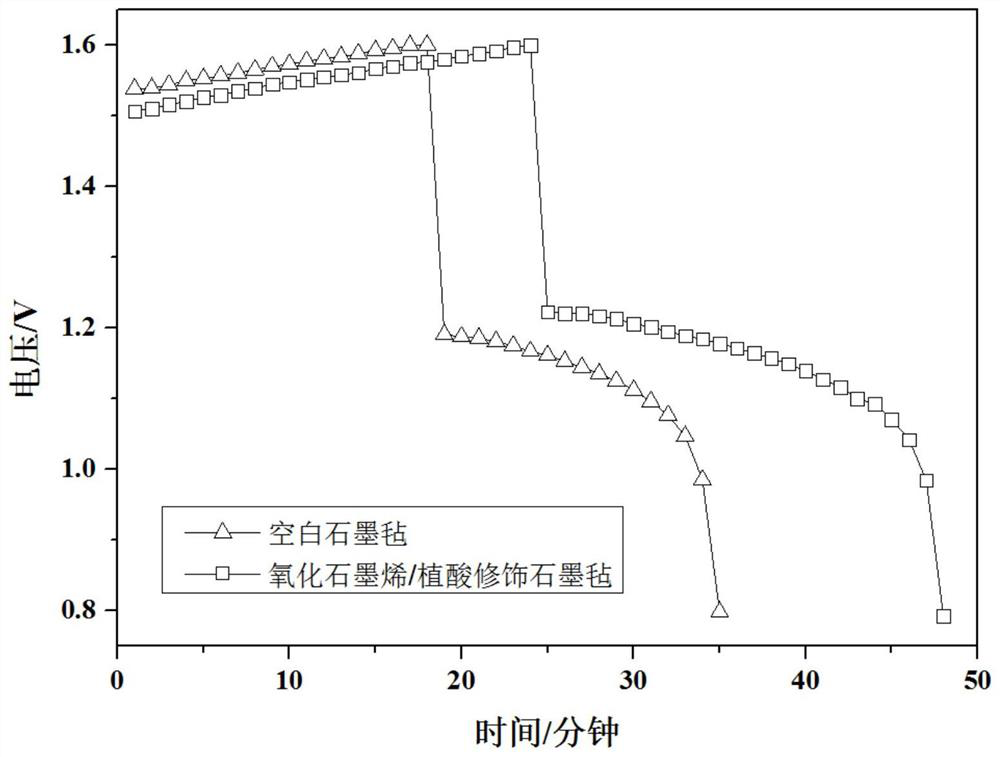
Electrode materials modified with superhydrophilic materials for energy storage flow batteries
A technology of electrode material and flow battery, which is applied in the direction of battery electrodes, fuel cells, regenerative fuel cells, etc., can solve the problems of cumbersome preparation process, high cost, and inapplicability to large-scale application of all-vanadium redox flow batteries, etc. Catalytic activity, improved electrical conductivity, beneficial effects on lateral transport and longitudinal migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] As mentioned above, the present invention provides a method for preparing an electrode material modified with a superhydrophilic material for an all-vanadium redox flow battery as described above, comprising the following steps:
[0038] Step 1: preparing graphene oxide solution;
[0039] Step 2: Put the graphene oxide solution obtained in step 1 into a reactor, add phytic acid solution, mix and stir evenly, add carbon-based materials, and ultrasonically mix to obtain a solid-liquid mixture;
[0040] Step 3: The solid-liquid mixture in step 2 is placed in a reactor for hydrothermal reaction, cooled to room temperature, and the electrode material modified by the superhydrophilic material is obtained.
[0041] In order to measure the high-temperature stability of the electrode material of the present invention, the electrode material obtained in step 3 can be placed in a tube furnace (which can be an argon atmosphere) or a muffle furnace after drying, and the electrode ma...
Embodiment 1
[0052] Take 0.3g of graphite powder with a purity of 99.9999%, grind it with an agate mortar for 30 minutes, mix 1.8g of potassium permanganate at the same time, add it to a beaker, add 36ml of concentrated sulfuric acid and 4ml of phosphoric acid, and keep the mixed solution at 35°C to 40°C for reaction 2 hours, stirred in a water bath at 50°C for 12 hours, cooled to room temperature, added 40ml of ice water, slowly added dropwise 3ml of 30% H 2 o 2 until no bubbles are formed. Put the above solution in a centrifuge tube, add 20ml of 30% HCl, centrifuge at 6000rpm for 20min, pour out the supernatant, repeat this process three times, add 20ml of deionized water to the centrifuge tube, centrifuge for 20min, and rotate at 8000r / min, repeat this process until it is washed to neutrality, place it in a beaker, add a certain amount of deionized water, ultrasonicate at room temperature for 2 hours, and centrifuge at 8000rpm for 20min to obtain graphene oxide precipitation.
[0053...
Embodiment 2
[0061] Put 0.5g of graphite powder and 0.375g of sodium nitrate in a beaker, add 37.5ml of concentrated sulfuric acid and stir in an ice bath, slowly add 2.2g of potassium permanganate, continue to stir in an ice bath for 2 hours, stir vigorously at room temperature for 5 days, add 70 mL 5wt%H 2 SO 4 Stir at 98°C for 1 hour, continue to stir at 98°C for 1 hour, add 2 mL of H 2 o 2 (30wt%) until no bubbles are generated, stir at room temperature for 2 hours, place the above solution in a centrifuge tube, add 20ml of 30% HCl, centrifuge at 6000rpm for 20min, pour out the supernatant, repeat this process three times, pour into the centrifuge tube Add 20ml of deionized water to the solution, centrifuge for 20 minutes, and rotate at 8000r / min. Repeat this process until the washing is neutral. Put it in a beaker, add a certain amount of deionized water, and ultrasonicate for 2 hours at room temperature. Centrifuge for 20min to obtain graphene oxide precipitate.
[0062] Take the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com