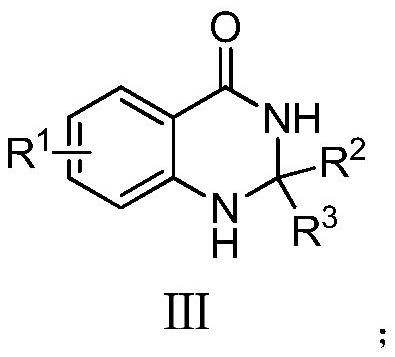
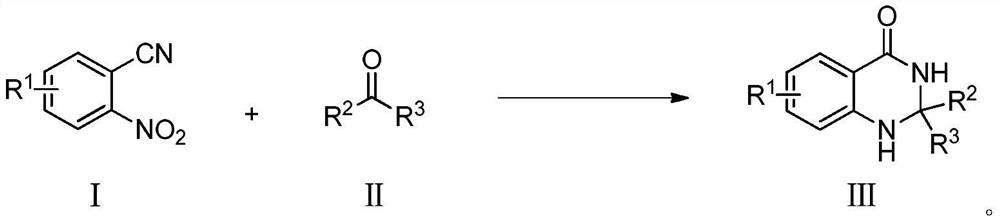
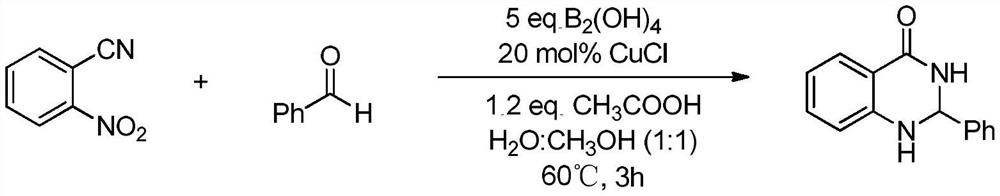Dihydroquinazolone compounds and preparation methods thereof
A technology for dihydroquinazolinones and compounds is applied in the field of dihydroquinazolinones and their preparation, and can solve the problems of harsh reaction conditions, single reaction raw materials, complicated post-processing processes and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] 2-Nitrobenzonitrile (0.2mmol, 29.6mg), tetrahydroxydiboron (1mmol, 89.6mg), glacial acetic acid (0.24mmol, 14.5mg), cuprous chloride (0.04mmol, 4.0mg), benzene Formaldehyde (0.24mmol, 25.4mg), methanol (1ml), and water (1ml) were sequentially added to the test tube, and reacted at 60°C for 3h. Analysis (petroleum ether: ethyl acetate = 4:1) separation, yield: (40.8mg, 91%). m.p.225-226℃. 1 HNMR (400MHz, CDCl 3 )δ=7.99(dd,J 1 =8.0Hz,J 2 =1.6Hz,1H),7.65(m,2H),7.50(m,2H),7.39(dt,J 1 =7.6Hz,J 2 =1.6Hz,1H),6.95(dd,J 1 =7.6Hz,J 2 =0.8Hz,1H),6.72(d,J=8.0Hz,1H),5.95(s,1H),5.80(s,1H),4.44(s,1H). 13 C NMR (100MHz, CDCl 3 )δ=164.7, 147.2, 138.6, 134.0, 130.2, 129.2, 128.8, 127.4, 119.7, 115.7, 114.6, 69.2.
Embodiment 2
[0026]
[0027] Add 2-nitrobenzonitrile (0.2mmol, 29.6mg), tetrahydroxydiboron (1mmol, 89.6mg), benzaldehyde (0.24mmol, 25.4mg), methanol (1ml), water (1ml) into the test tube in turn , reacted at 60°C for 12h, after the end, the reaction solution was extracted 3 times with ethyl acetate, the combined organic phase was concentrated to dryness, and separated by column chromatography (petroleum ether: ethyl acetate = 4:1), the yield: (6.7mg, 15%).
Embodiment 3
[0029]
[0030] 2-Nitrobenzonitrile (0.2mmol, 29.6mg), tetrahydroxydiboron (1mmol, 89.6mg), glacial acetic acid (0.24mmol, 14.5mg), cuprous chloride (0.04mmol, 4.0mg), benzene Add formaldehyde (0.24mmol, 25.4mg) and water (2ml) into the test tube in turn, and react at 60°C for 3h. Ethyl acetate=4:1) isolated, yield: (13.2 mg, 30%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com