Green fluorescent material and preparation method thereof
A technology of green fluorescence and raw materials, applied in the field of fluorescent materials, can solve the problems of harsh sintering temperature, not many fluorescent materials, and not easy to excite, etc., and achieve the effect of simple and mild preparation method, high chemical and thermal stability, and simple and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
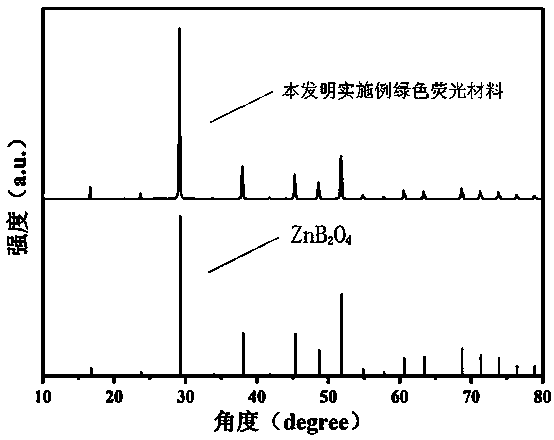
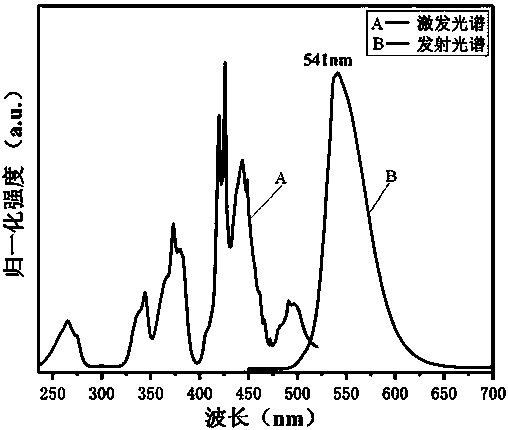
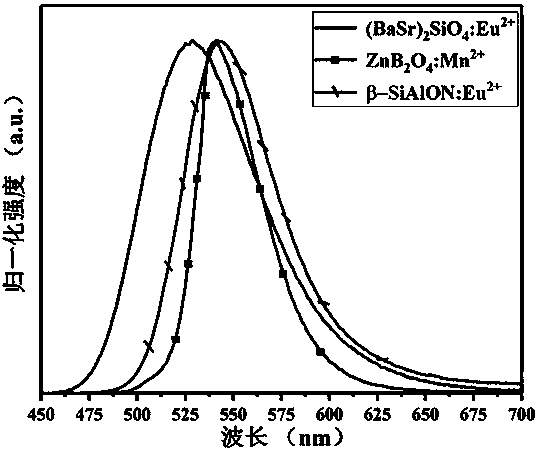
[0038] A green fluorescent material whose chemical expression is Zn 0.95 B 2 o 4 : 0.05Mn 2+ . The raw material is H 3 BO 3 , ZnO and (CH 3 COO) 2 Mn•4H 2 O, the preparation method of this green fluorescent material specifically comprises the following steps:
[0039] (1) According to the stoichiometric ratio of each chemical composition in the above chemical expression, weigh 61.833g H 3 BO 3 , 38.669g ZnO, 6.147g (CH 3 COO) 2 Mn•4H 2 O as raw material.
[0040] Specifically, the weight and proportion of raw materials are calculated by the following chemical reaction formula:
[0041] h 3 BO 3 +0.95ZnO+0.05(CH 3 COO) 2 Mn•4H 2 O=Zn 0.95 mn 0.05 B 2 o 4 +1.1H 2 O+0.1CO 2
[0042] Among them, H 3 BO 3 The relative molecular mass of ZnO is 61.8330, the relative molecular mass of ZnO is 81.4084, (CH 3 COO) 2 Mn•4H 2 The relative molecular mass of O is 245.0872. The mass of the above raw materials can be calculated according to the molar ratio of the...
Embodiment 2
[0066] A green fluorescent material whose chemical expression is Zn 0.8 B 2 o 4 : 0.2Mn 2+ . The raw material is B 2 o 3 , Zn(NO 3 ) 2 •6H 2 O and MnCO 3 , the preparation method of the green fluorescent material specifically includes the following steps:
[0067] (1) According to the stoichiometric ratio of each chemical composition in the above chemical expression, weigh 34.81g B 2 o 3 , 119.00gZn(NO 3 ) 2 •6H 2 O, 11.49 g MnCO 3 as raw material.
[0068] (2) Same as embodiment 1.
[0069] (3) The raw material powder is placed in an environment with a temperature of 800° C., and calcined in a nitrogen atmosphere for 8 hours.
[0070] (4), (5) are all the same as embodiment 1.
[0071] So far, 74.47g of green fluorescent material Zn 0.8 B 2 o 4 : 0.2Mn 2+ .
Embodiment 3
[0073] A green fluorescent material whose chemical expression is Zn 0.7 B 2 o 4 : 0.3Mn 2+ . The raw material is H 3 BO 3 , (CH 3 COO) 2 Zn•2H 2 O and MnC 2 o 4 , the preparation method of the green fluorescent material specifically includes the following steps:
[0074] (1) According to the stoichiometric ratio of each chemical composition in the above chemical expression, weigh 61.83g H 3 BO 3 , 76.83g (CH 3 COO) 2 Zn•2H 2 O and 21.44gMnC 2 o 4 as raw material.
[0075] (2) Same as embodiment 1.
[0076] (3) The raw material powder is placed in an environment with a temperature of 900° C., and calcined in a nitrogen atmosphere for 4 hours.
[0077] (4) and (5) are the same as in Example 1, so as to prepare a green fluorescent material.
[0078] So far, 73.94g of green fluorescent material Zn 0.7 B 2 o 4 : 0.3Mn 2+ .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com