Method for improving surface conductivity of nickelous hydroxide
A nickel hydroxide and conductivity technology, which is applied in the field of improving the surface conductivity of nickel hydroxide, can solve the problems of uneven distribution of CoOOH and insufficient contact, and achieve suitable large-scale operation, improved conductivity, specific capacity and high current The effect of improving charge and discharge capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
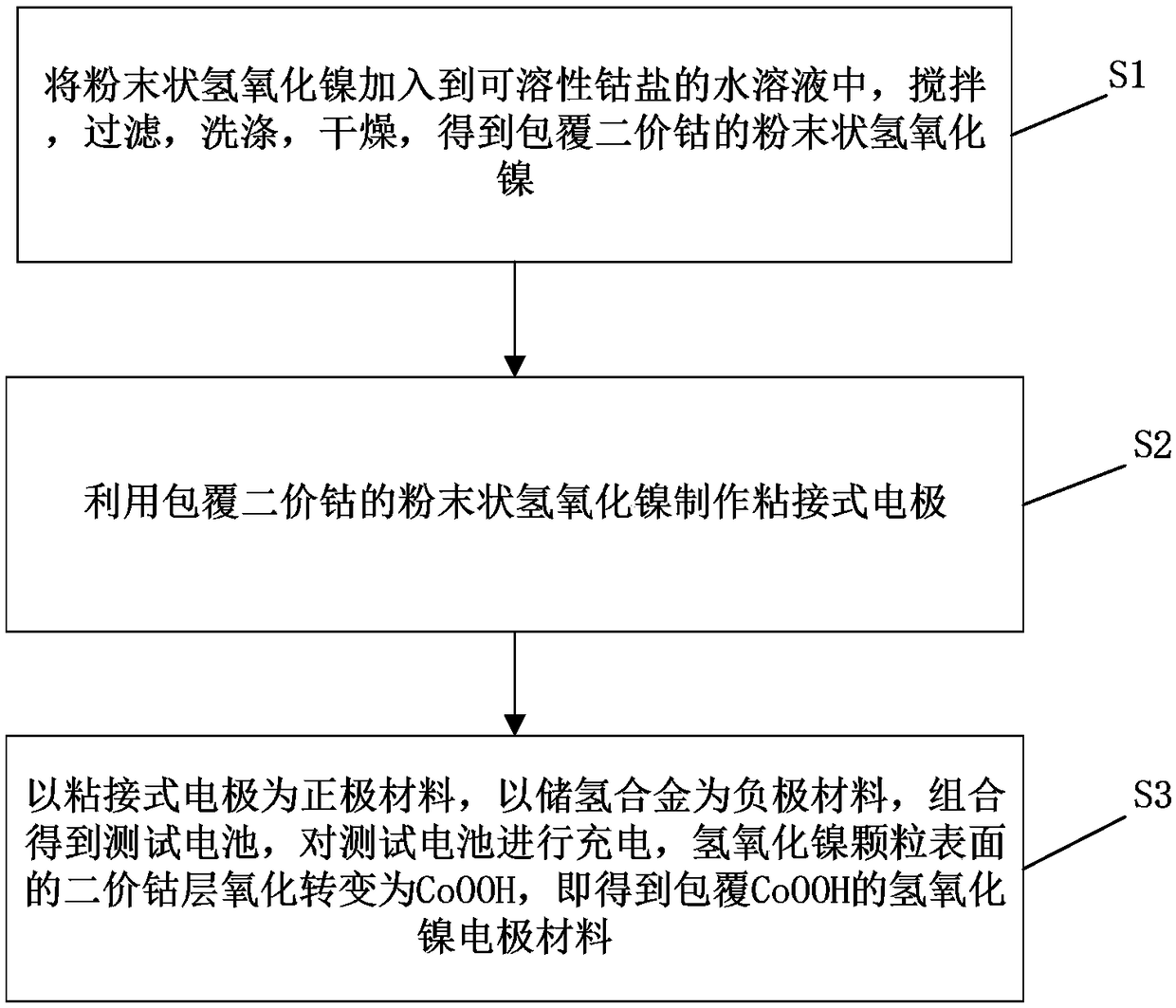
[0029] First, dissolve 0.728g of cobalt nitrate hydrate in 50mL of distilled water in the reactor, add 5g of powdered nickel hydroxide, place the reactor in a water bath at 50°C, keep stirring for 10h, cool naturally, filter, and wash with pure water Dry at 80°C for 5 hours to obtain powdered nickel hydroxide coated with divalent cobalt; mix the powdered nickel hydroxide coated with divalent cobalt with polytetrafluoroethylene emulsion in a mass ratio of 100:5 and add Appropriate amount of deionized water, mechanically stirred and mixed thoroughly to form a slurry, and then the slurry was coated on the foamed nickel current collector, dried at 80°C for 4 hours, and compacted by a roller press to obtain a bonded electrode. The test battery was composed of a formula electrode and a hydrogen storage alloy negative electrode, and then charged at a constant current of 0.05C for 20 hours, so that the divalent cobalt on the surface of the nickel hydroxide particles was oxidized during...
Embodiment 2
[0036] The only difference between this example and Example 1 is that in the reactor, 0.387g of cobalt sulfate was dissolved in 50mL of distilled water, and after adding 5g of powdered nickel hydroxide, the reactor was placed in a water bath at 48°C, and kept stirring for 9.5h , filtered after natural cooling, washed with pure water and dried at 75°C for 4 hours; the rest were basically the same as in Example 1.
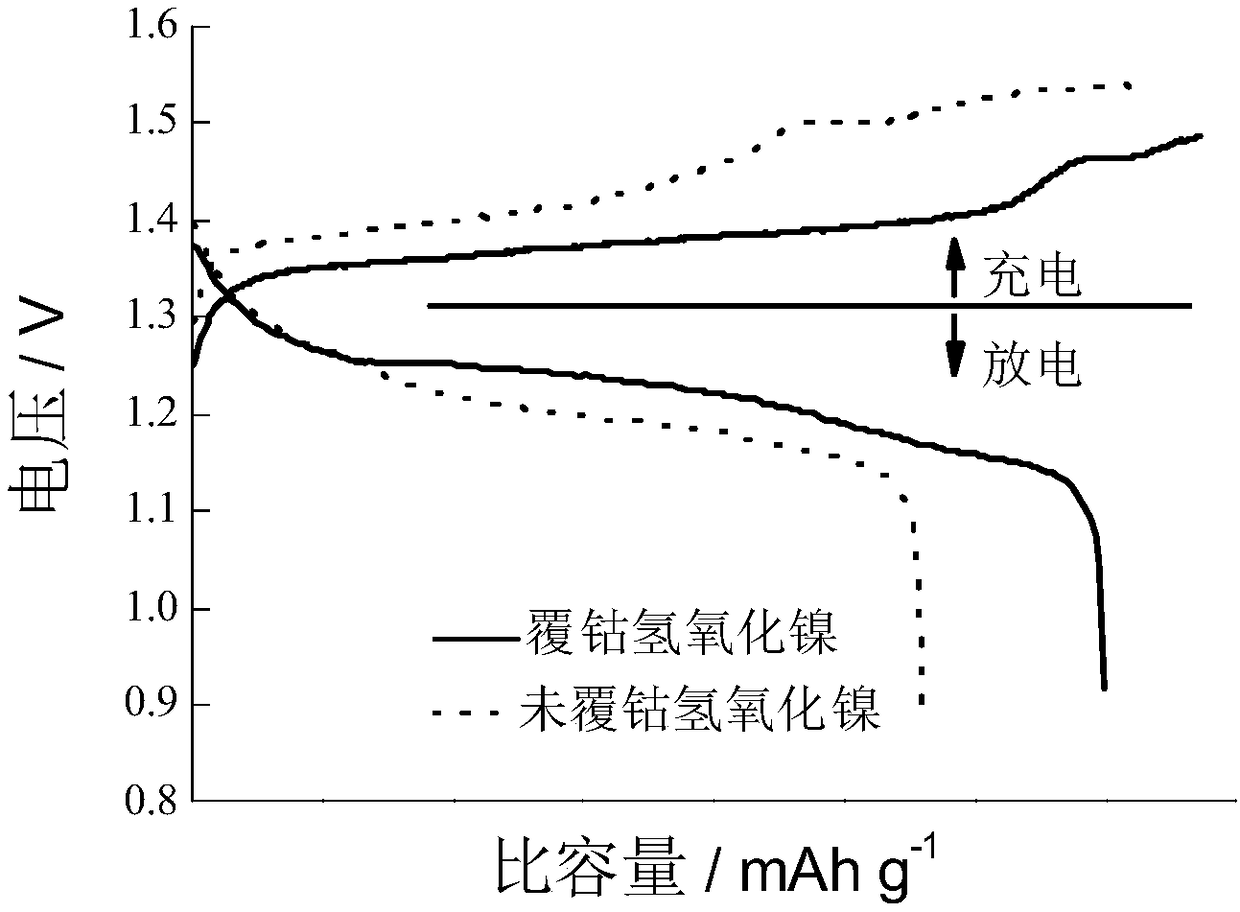
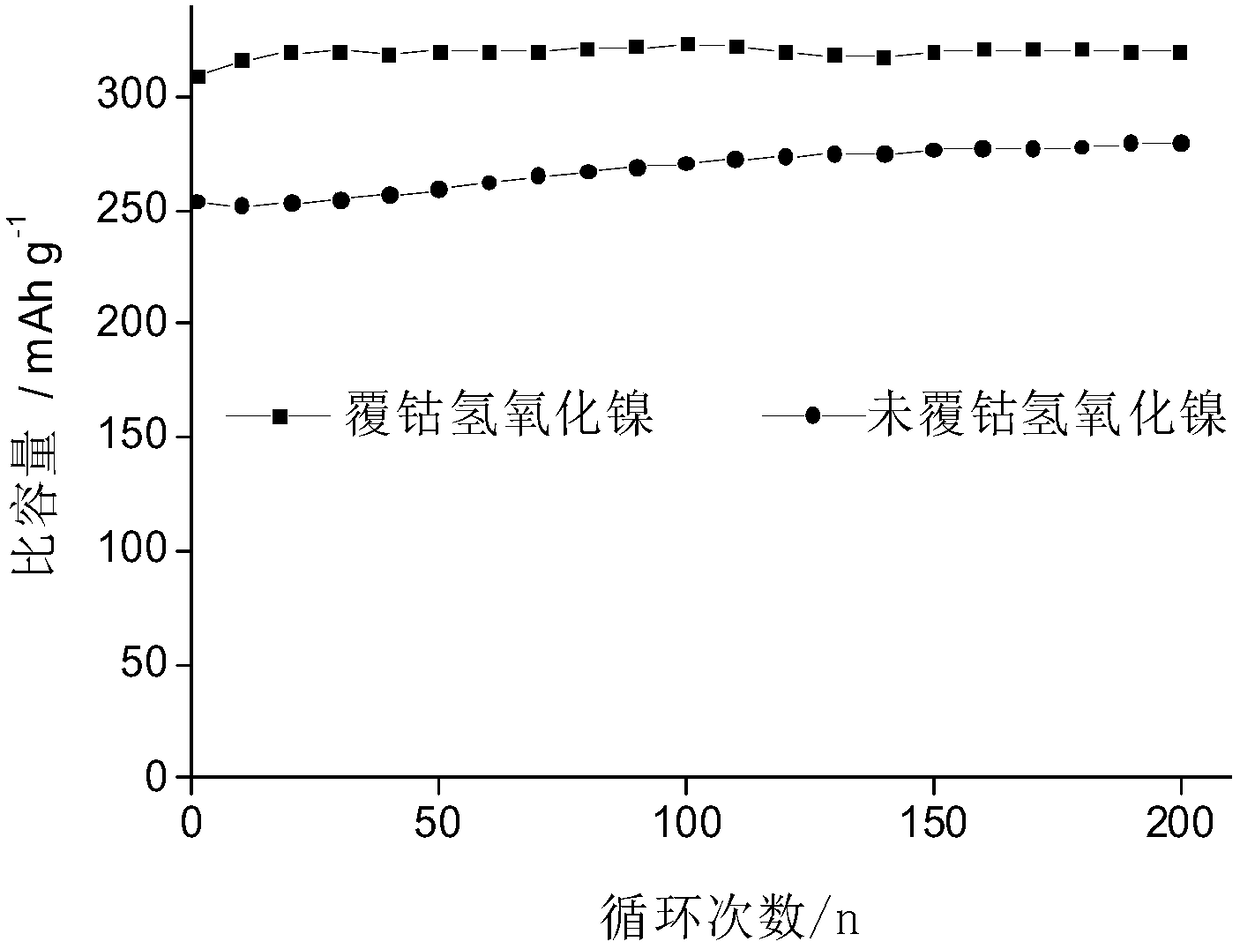
[0037] Activation and charge-discharge tests were performed on the CoOOH-coated nickel hydroxide electrode material prepared in Example 2. The results showed that the 1C discharge specific capacity of the CoOOH-coated nickel hydroxide electrode prepared in Example 2 was 310mAh / g.
Embodiment 3
[0039] The only difference between this example and Example 1 is that in the reactor, 0.595g of cobalt chloride hydrate was dissolved in 50mL of distilled water, and after adding 5g of powdered nickel hydroxide, the reactor was placed in a water bath at 52°C and kept stirring 10.5h, filtered after natural cooling, washed with pure water and dried at 85°C for 5.5h; the rest were basically the same as in Example 1.
[0040] Activation and charge-discharge tests were performed on the CoOOH-coated nickel hydroxide electrode material prepared in Example 3. The results showed that the 1C discharge specific capacity of the CoOOH-coated nickel hydroxide electrode prepared in Example 3 was 308mAh / g.
[0041] The unreacted cobalt in the reaction solution involved in the method provided by the present invention can be recovered by a simple method and reintroduced into the system for use.
[0042] The method provided by the invention first utilizes the ion exchange reaction between the di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com