Phenyl bridge bond type fibrous porous silicon material and preparation method and application thereof
A fibrous, porous silicon technology, applied in the direction of material separation, analytical materials, chemical instruments and methods, etc., can solve problems such as residues, ecosystems and human health hazards, and achieve small amount of organic solvents, good adsorption performance, and high mechanical properties. Effect of Strength and Chemical Stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
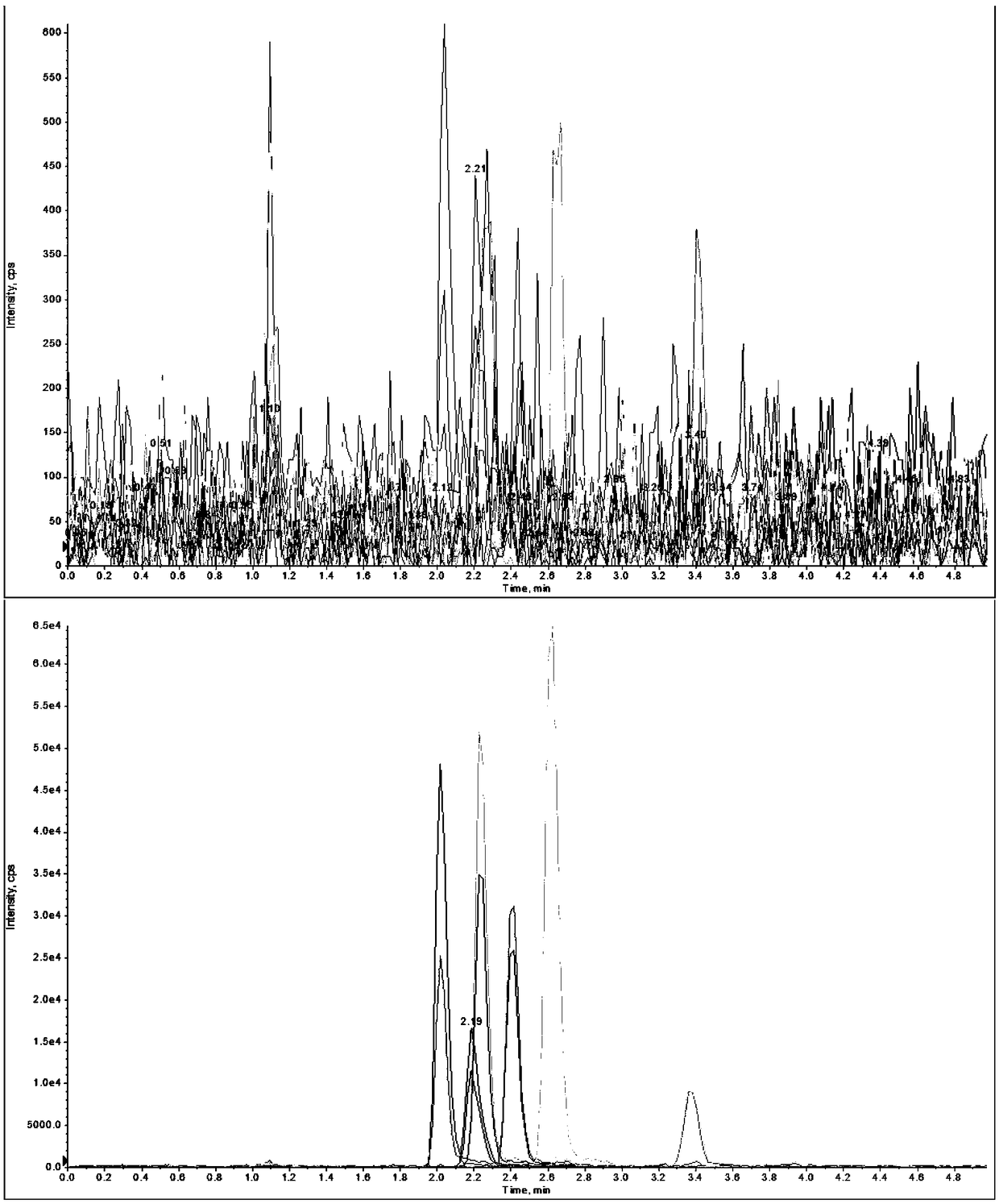
[0029] Example 1. The recovery rate experiment of sulfa antibiotics in purified water
[0030] (1) Preparation of phenyl bridged fibrous porous silicon solid-phase extraction column:
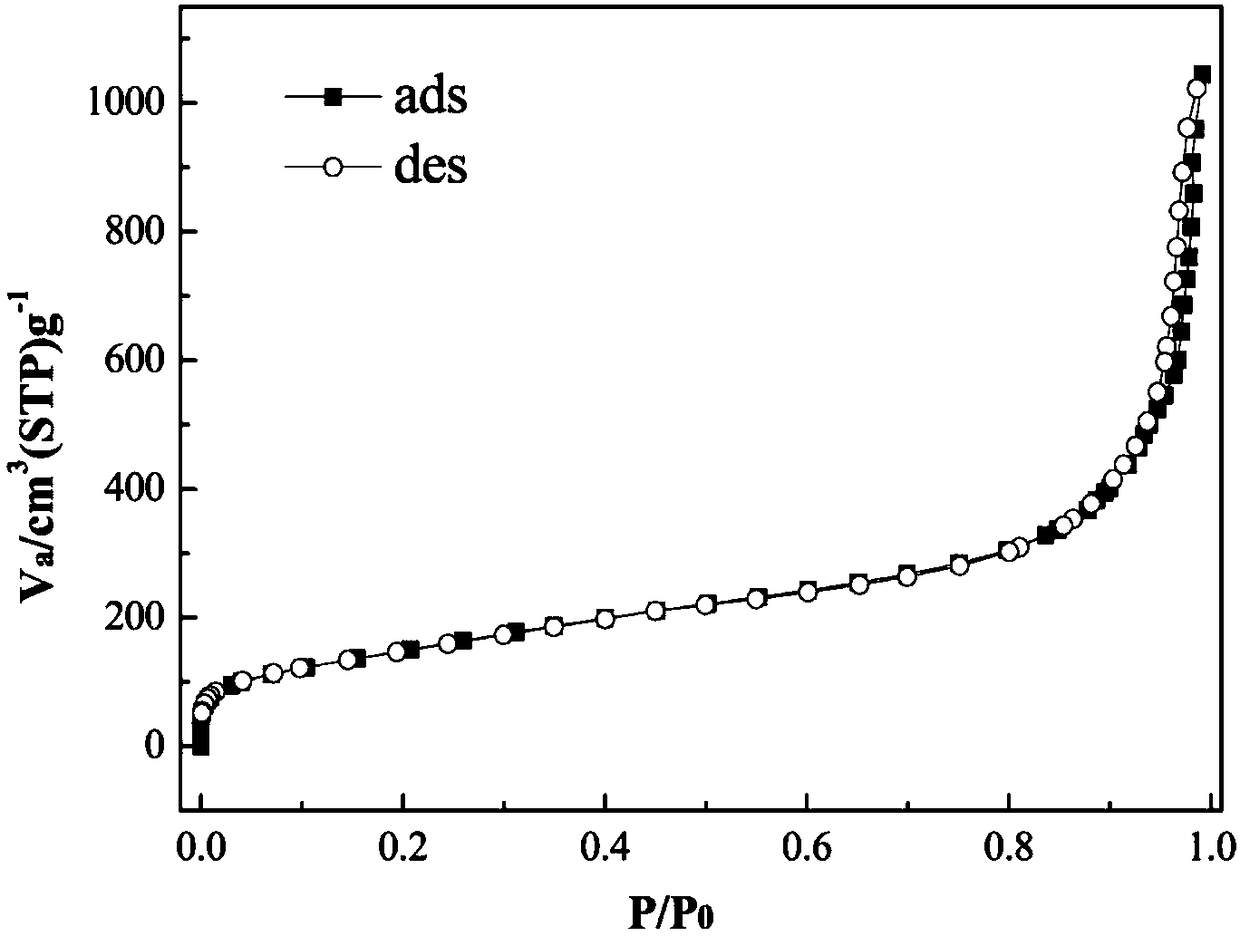
[0031] Weigh 1.0 g of cetylpyridinium bromide, add 0.6 g of urea into 30 mL of water, and stir for 0.5 hour to obtain solution A. Add bis(triethoxysilyl)benzene / ethylorthosilicate and 1.5 mL amyl alcohol in a molar ratio of 1:6 to 30 mL cyclohexane and stir for 0.5 hour to obtain solution B. Add solution B to solution A, stir in a water bath at room temperature for 1 hour, then transfer to a 100 mL polytetrafluoroethylene reactor, and react at 120°C for 6 hours. After centrifugation and washing, a white powder was obtained. In an ethanol solution containing 1% of 36% hydrochloric acid, reflux for 24 hours, remove the surfactant, centrifuge, wash, and dry to obtain a phenyl bridged fibrous porous silicon filler. The morphology of phenyl bridged fibrous porous silicon is characterized by fiber ...
Embodiment 2
[0037] Example 2. The recovery rate experiment of sulfa antibiotics in milk
[0038] (1) Preparation of phenyl bridged fibrous porous silicon solid-phase extraction column:
[0039] Using dodecylpyridinium chloride as a structure-directing agent, the silicon source precursor is adjusted to bis(trimethoxysilylethyl)benzene and methyl orthosilicate with a molar ratio of 1:4, using the method of Example 1 The material synthesis step is to prepare phenyl-bridged fibrous porous silicon. A 3 mL empty column tube was filled with 60 mg of phenyl bridged fibrous porous silicon, and compacted with a sieve plate with a thickness of 1.0 mm and a pore diameter of 50 μm to obtain a solid phase extraction column. Pretreat with methanol and water before use, that is, rinse the cartridge with 5mL of methanol first, and then rinse the cartridge with 5mL of water.
[0040] (2) Extraction analysis process of milk with phenyl bridged fibrous porous silicon solid-phase extraction column:
[0041...
Embodiment 3
[0044] Example 3. The standard recovery experiment of sulfa antibiotics in honey
[0045] (1) Preparation of phenyl bridged fibrous porous silicon solid-phase extraction column:
[0046] Using cetyltrimethylammonium bromide as a structure-directing agent, the silicon source precursor was adjusted to [1,4-phenylenebis(methylene)]bis(trimethoxyl) with a molar ratio of 1:2 Silane) and butyl orthosilicate, using the same material synthesis steps as in Example 1, to prepare phenyl bridged fibrous porous silicon. A 10 mL empty column tube was filled with 200 mg of phenyl bridged fibrous porous silicon, and compacted with a sieve plate with a thickness of 2.5 mm and a pore diameter of 20 μm to obtain a solid phase extraction column. Pretreat with methanol and water before use, that is, rinse the cartridge with 5mL of methanol first, and then rinse the cartridge with 5mL of water.
[0047] (2) Extraction and analysis process of honey with phenyl bridge type fibrous porous silicon so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com