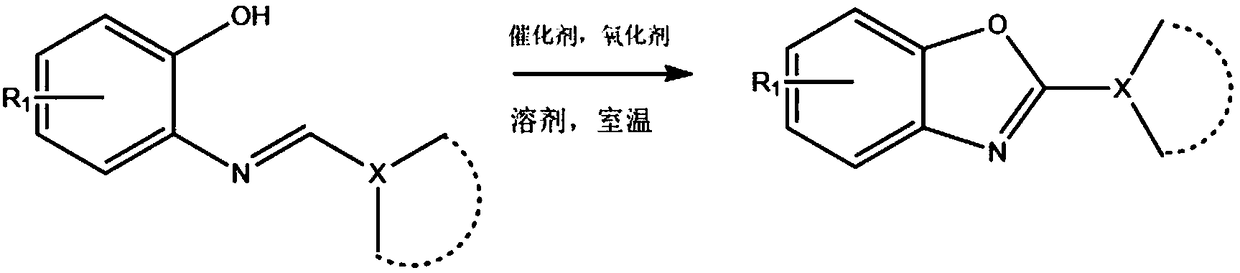
Green method of synthesizing 2-substituted benzoxazole compounds through biocatalysis oxidation
A technology of benzoxazole and biocatalysis, applied in the direction of organic chemistry, can solve the problems of harmful and expensive curing agents, and achieve the effect of reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] In 2ml of dichloromethane, use hemoglobin as a catalyst (0.05mol% or 0.5μmol), and in the case of tert-butyl hydroperoxide as an oxidizing agent (double amount), add 110μl of benzaldehyde and 109.0mg of o-aminophenol to prepare The intermediate was stirred at room temperature for 2h. The reaction progress was detected by thin-layer chromatography, and after the reaction, the organic layer was dried and concentrated with anhydrous Na2SO4. Then it was further purified by silica gel column chromatography (ethyl acetate / hexane) to obtain 175.5 mg of 2-phenylbenzoxazole; the yield was 90%. White solid.1H NMR (500MHz, CDCl3) δ8.32–8.30(m,2H),7.83–7.81(m,1H), 7.63-7.62(m,1H),7.58-7.55(m,3H),7.40( m,2H)ppm;
Embodiment 2
[0030]
[0031] Using hemoglobin as a catalyst (0.05mol% or 0.5μmol) in 2ml of dichloromethane, in the case of tert-butyl hydroperoxide as an oxidizing agent (double amount), add p-tolualdehyde 130μl and o-aminophenol 109.0 mg The reaction was stirred at room temperature for 2h. The reaction progress was detected by thin-layer chromatography, and after the reaction, the organic layer was dried and concentrated with anhydrous Na2SO4. Then, it was further purified by silica gel column chromatography (ethyl acetate / hexane) to obtain 198.6 mg of 2-p-tolylbenzoxazole; the yield was 95%. 1H NMR (500 MHz, CDCl3) δ8.16(d, J=5.0Hz, 2H), 7.79–7.75(m,1H), 7.59–7.56(m,1H), 7.35(m,4H), 2.45(s ,3H)ppm;
Embodiment 3
[0033]
[0034] Using hemoglobin as a catalyst (0.05mol% or 0.5μmol) in 2ml of dichloromethane, in the case of tert-butyl hydroperoxide as an oxidizing agent (double amount), add 130μl of p-methoxybenzaldehyde and o-aminophenol 109.0 mg of the prepared intermediate was stirred and reacted at room temperature for 2 h. The reaction progress was detected by thin-layer chromatography, and after the reaction, the organic layer was dried and concentrated with anhydrous Na2SO4. Then, it was further purified by silica gel column chromatography (ethyl acetate / hexane) to obtain 209.2 mg of 2-p-methoxyphenylbenzoxazole; the yield was 93%. White solid. 1H NMR (500MHz, CDCl3) δ8.18 (d, J = 5.0Hz, 2H), 7.71 -7.70 (m, 1H), 7.53 (m, 1H), 7.28 (m, 2H), 6.99 (m ,2H),3.86(s,3H) ppm;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com