Preparation method of 3-aminofurazan-4-methanamide
A technology of aminofurazan and formamide, which is applied in the field of organic intermediate synthesis, can solve the problems of poor reaction selectivity, low yield, cumbersome synthesis steps, etc., and achieve easy-to-obtain raw materials, high purity and yield, and simple process flow Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The first step: the synthesis of 2-oximino cyanoacetamide (2)
[0032]
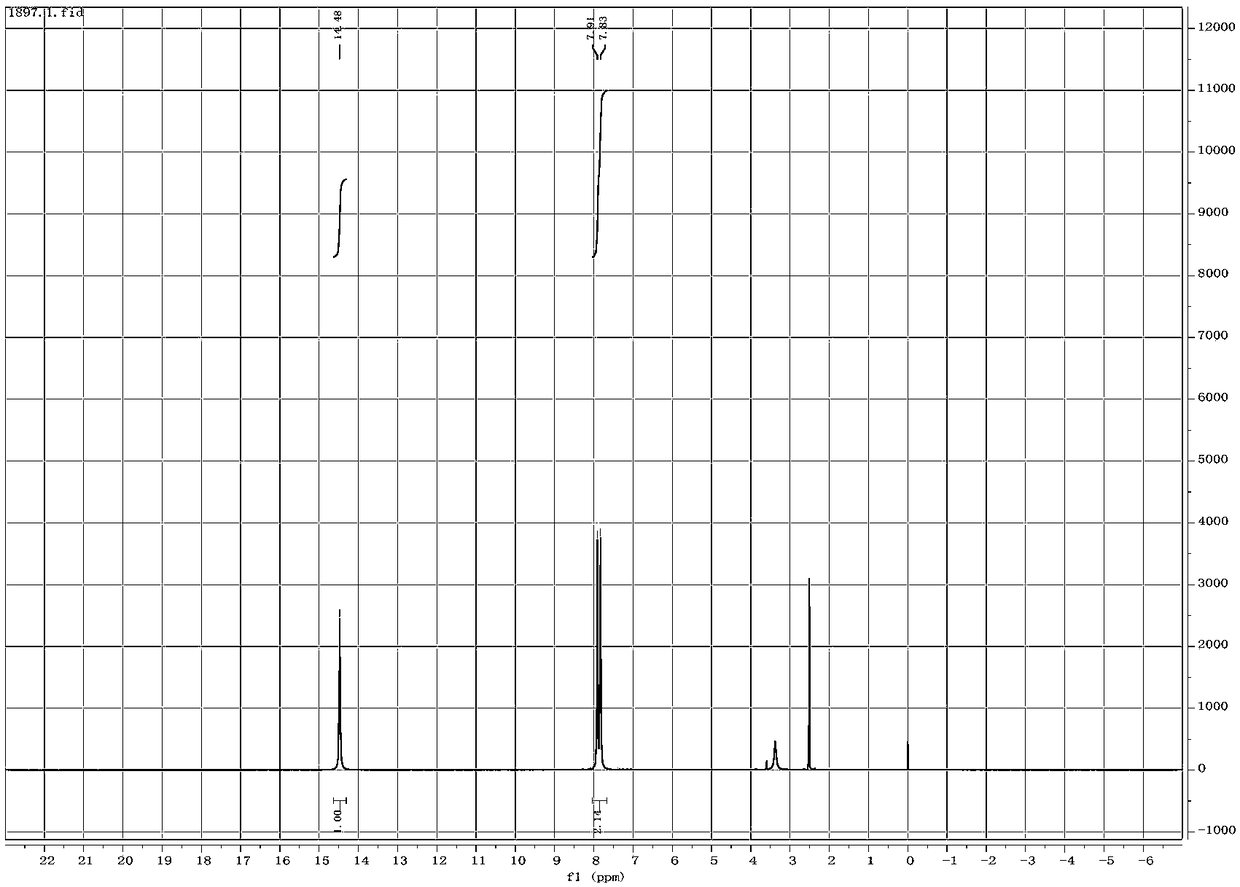
[0033] Add 100mL of deionized water into a 250mL reaction flask, then weigh cyanoacetamide 1 (12.61g, 0.15mol) and sodium nitrite (12.42g, 0.18mol) into the reaction flask in turn, add ice Acetic acid (17.1 mL, 0.3 mol) was dropped, stirred at room temperature for 10 h, and the reaction progress was tracked by TLC. After the reaction was completed, a white filter cake was obtained by suction filtration, and dried in an oven to obtain a white powder (14.48 g, molar yield 85.4%).
[0034] Melting point: 171.8°C-172.0°C
[0035] 1 H NMR (500MHz, DMSO-d 6 )δ: 7.83, 7.91 (2br s, 2H, CON H 2 ),14.48(s,1H,O H ).
[0036] The second and third one-pot synthesis: Synthesis of 3-aminofurazan-4-carboxamide (4)
[0037]
[0038] Add 3.6mL of anhydrous methanol in the dry reaction flask, weigh the methanol solution (1.47g, 7.5mmol) of sodium methoxide (1.47g, 7.5mmol) and hydroxylamine hydrochloride ...
Embodiment 2
[0040] The first step: the synthesis of 2-oximino cyanoacetamide (2)
[0041] Add 63mL of deionized water into a 250mL reaction flask, then weigh cyanoacetamide 1 (12.61g, 0.15mol) and sodium nitrite (11.38g, 0.16mol) into the reaction flask in turn, add formic acid dropwise under ice-water bath conditions (8.5mL, 0.22mol), after dropping, stir at room temperature for 8h, and track the reaction progress by TLC. After the reaction was completed, a white filter cake was obtained by suction filtration, and dried in an oven to obtain a white powder (14.07 g, molar yield 83.0%).
[0042] The second and third one-pot synthesis: Synthesis of 3-aminofurazan-4-carboxamide (4)
[0043] Add 4.6mL of anhydrous methanol to the dry reaction flask, weigh the ethanol solution (3.20g, 10mmol) of sodium ethoxide (3.20g, 10mmol) and hydroxylamine hydrochloride (0.70g, 10mmol) with a mass fraction of 21% and drop into the reaction flask in turn, and stir for 0.5h . Then add 2 (0.57g, 5mmol), a...
Embodiment 3
[0045] The first step: the synthesis of 2-oximino cyanoacetamide (2)
[0046] Add 113mL of deionized water into a 250mL reaction flask, then weigh cyanoacetamide 1 (12.61g, 0.15mol) and sodium nitrite (10.35g, 0.15mol) into the reaction flask in turn, add the mass of Sulfuric acid (11.9 mL, 0.15 mol) with a fraction of 75% was added dropwise, stirred at room temperature for 12 h, and the reaction progress was tracked by TLC. After the reaction was completed, a white filter cake was obtained by suction filtration, and dried in an oven to obtain a white powder (13.92 g, molar yield 82.1%).
[0047] The second and third one-pot synthesis: Synthesis of 3-aminofurazan-4-carboxamide (4)
[0048] Add 11mL of anhydrous tert-butanol to the dry reaction flask, weigh sodium tert-butoxide (0.48g, 5mmol) and hydroxylamine hydrochloride (0.35g, 5mmol) into the reaction flask in turn, and stir for 0.5h. Then add 2 (0.57g, 5mmol), and stir at 85°C for 5h. After the reaction is completed, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com