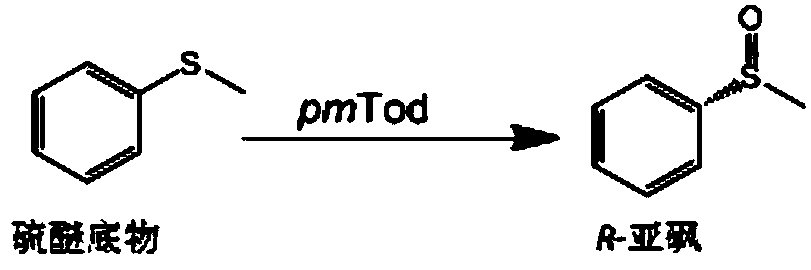
Monooxygenase complex and application thereof in chiral sulfoxide synthesis
A technology of monooxygenase and complex, which is applied in the field of molecular biology, can solve the problems of lack of chiral sulfoxide synthase and cannot meet the needs of green industrial production, and achieve the effects of environmental friendliness, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the acquisition of monooxygenase complex gene, comprises the following steps:
[0027] Using genomic DNA as a template, use primers 1: 5'-GGCCAGATCTCATGAATCAGACCGACACATC-3' and 2: 5'-AATTCTCGAGGCGTGTCGCCTTCAGCGCG-3' for PCR amplification to obtain the pmTodA gene; use primers 3: 5'-GGAAGGATCCGATGATTGATTCAGCCAACAG-3' and 4: 5 '-AACCGTCGACCTAGAAGAAGAAACTGAGG-3' was used for PCR amplification to obtain pmTodB gene; use primer 5: 5'-GGCCAGATCTATGGCGTTGCCCGGCAGCC-3' and 6: 5'-AATTCTCGAG ACGTTAGGTCTCCTTCATTCG-3' for PCR amplification to obtain pmTodC gene; use primer 7: 5' -GGAAGGATCCATGACTTGGACATACATATT-3' and 8:5'-AACCGTCGACCTTCAACTCCCCGTTGTCG-3' were amplified by PCR to obtain the pmTodD gene. The PCR reaction system was as follows: 10.0 μL of 2×Taq PCR Master Mix, 1 μL of genomic DNA, 0.5 μL of upstream and downstream primers, and 8.0 μL of ddH2O. PCR reaction conditions: 95°C for 10 min; 98°C for 10 s, 58°C for 30 s, 72°C for 30 s, 30 cycles; 72°C for 5 mi...
Embodiment 2
[0028] Embodiment 2: the construction of monooxygenase complex genetically engineered bacterium, comprises the following steps:
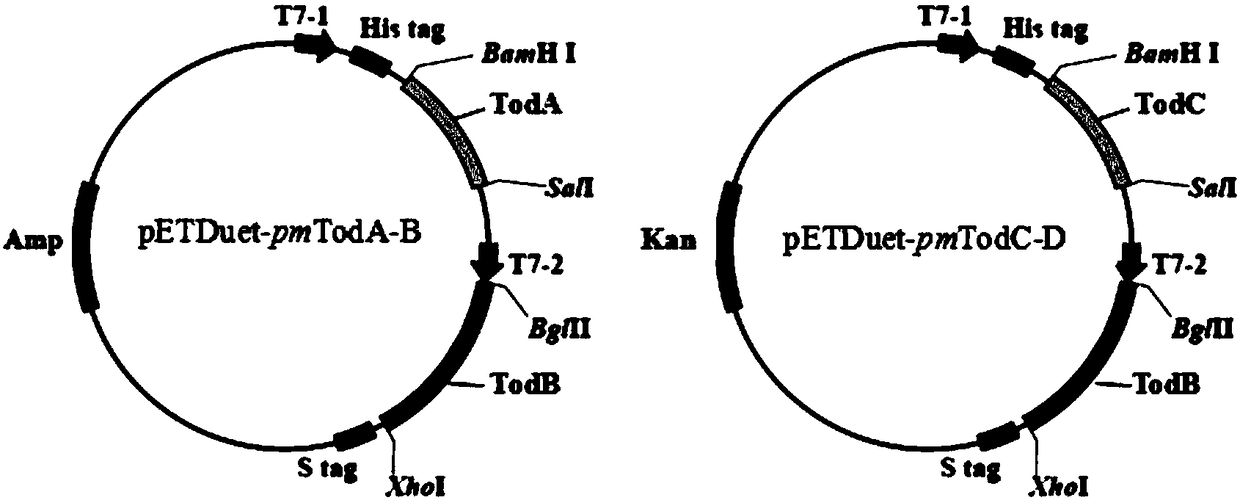
[0029] The DNA fragment containing the pmTodA gene sequence and the vector pETDute-1 were double digested with restriction endonucleases BamH I and Sal I, and the recovered target fragment and vector were digested. Using T4 DNA ligase, after ligation at 16°C for 4 hours, the ligation product was transformed into Escherichia coli DH5α. After overnight culture, the grown monoclonal colony was picked and shaken to extract the plasmid, and the recombinant plasmid pETDute1-pmTodA was detected by double enzyme digestion with BamH I and Sal I. Next, after performing the same operation on the pmTodB gene and the recombinant plasmid pETDute1-pmTodA with Bgl II and Xho I, the recombinant vector pETDute1-pmTodA-B was obtained. Next, the DNA fragment containing the pmTodC gene sequence and the vector pRSFDute-1 were double-digested with restriction enzymes Bam...
Embodiment 3
[0030] Embodiment 3: the acquisition of monooxygenase complex recombinant protein, comprises the following steps:
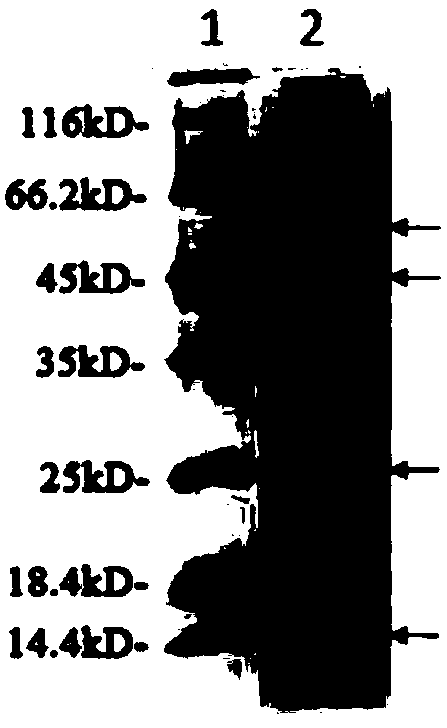
[0031] After activating the genetically engineered bacteria BL21 (DE3) stored in glycerol containing recombinant plasmids, pick a single colony in 2ml of liquid LB medium containing the corresponding antibiotics, shake and culture at 37°C for 12 hours, and transfer to 2% inoculum the next day. Inoculated in fresh 100mL LB liquid medium containing antibiotics, 37°C 250rpm shaking culture OD600 was 0.6 (about 3h), added IPTG with a final concentration of 0.2mM, 25°C 160rpm induction culture for 8h. After the induction, collect the cells by centrifugation at 5000rpm / min for 5min, resuspend the cells in PBS buffer, break up the cells by ultrasonic, and centrifuge at 15000rpm for 5min to remove cell debris, take the supernatant and mix it with 2x loading buffer, and put it in a constant temperature metal bath SDS-PAGE electrophoresis analysis was performed after heati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com