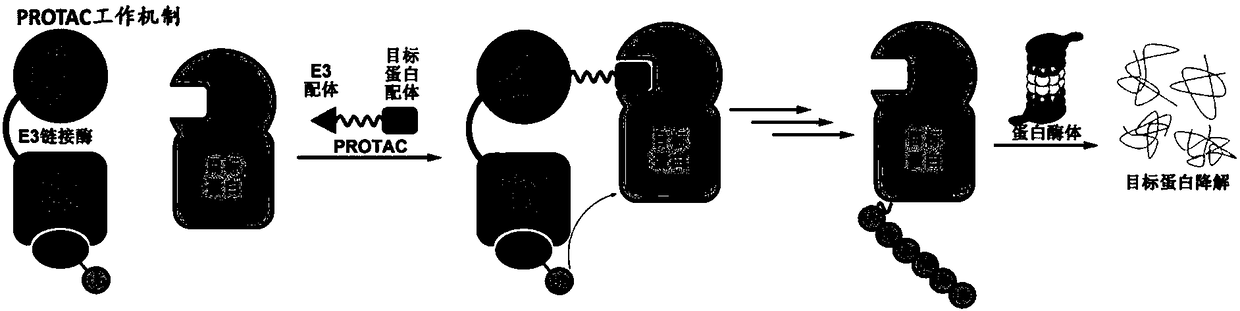
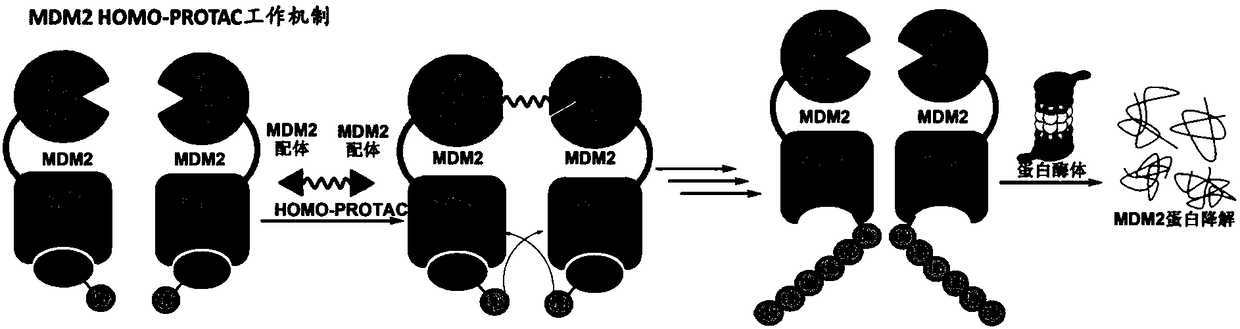
Dimer ester micromolecule PROTACs for inducing MDM2 to self-degrade E3 ubiquitin ligase
A technology of enzyme dimer and MDM2, applied in the direction of organic active ingredients, medical preparations containing active ingredients, organic chemistry, etc., to achieve the best anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0096] The preparation method of PROTACs of any structure as above comprises the following steps:
[0097] (a) piperazin-2-one is substituted after group protection, and then deprotected to obtain
[0098] In this step, the imino group at the 4-position of the raw material piperazin-2-one is first protected, and common protecting agents such as Cbz, Boc, Fmoc, Alloc, Teoc, or Tos and TMB can be used for group protection;
[0099] Then, the group-protected product was combined with BrCH 2 COOR 14 Reaction to replace the amino group at position 1;
[0100] Then, deprotection is carried out, and the protecting group is removed to obtain compound (i);
[0101] In step (a), R 14 C1-C6 straight chain or branched chain alkyl with or without substituents, such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl, tert-butyl, pentyl, Isopentyl, neopentyl, or hexyl, etc.
[0102] (b) Substitution of 2,4-dihydroxybenzaldehyde to give
[0103] In this step, the target product i...
Embodiment 1
[0135] Taking the preparation of 15 with a symmetrical structure as an example, the preparation method of PROTACs of the present invention is introduced as follows:
[0136] Synthesis of tert-butyl 3-oxopiperazine-1-carboxylate (1)
[0137] Piperazin-2-one (1.0 g, 10 mmol) was suspended in 10 mL of DCM, Boc was added slowly 2 O. After the addition was complete, it was left overnight at room temperature. After the reaction, wash three times with 0.1N dilute hydrochloric acid, 20ml each time, and wash three times with saturated potassium carbonate 20ml each time. Finally the dichloromethane was dried over anhydrous sodium sulfate and the solution was removed in vacuo to give a white solid.
[0138] Product melting point: 161-162°C, 1 H NMR (400MHz, CDCl 3 )δ7.66(s,1H),4.07(s,2H),3.62(t,J=5.3Hz,2H),3.37(s,2H),1.47(s,9H).
[0139] Synthesis of tert-butyl 4-(2-methoxy-2-oxoethyl)-3-oxopiperazine-1-carboxylate (2)
[0140] In a 500 mL three-necked flask, 12.70 g (63.0 mmol, 1...
experiment example 1
[0171] In order to test the effect of the compound of the present invention, the inventors conducted experimental tests on the effect of compound 15a in A549 cells.
[0172] The specific experimental method is as follows: A549 cells were treated with 20 μM compound 15a for 12 hours; at the same time, a sample without compound was prepared as a negative control.
[0173] After treatment, cells were lysed and subjected to immunoblot analysis to monitor changes in the levels of endogenous MDM2 and p53.
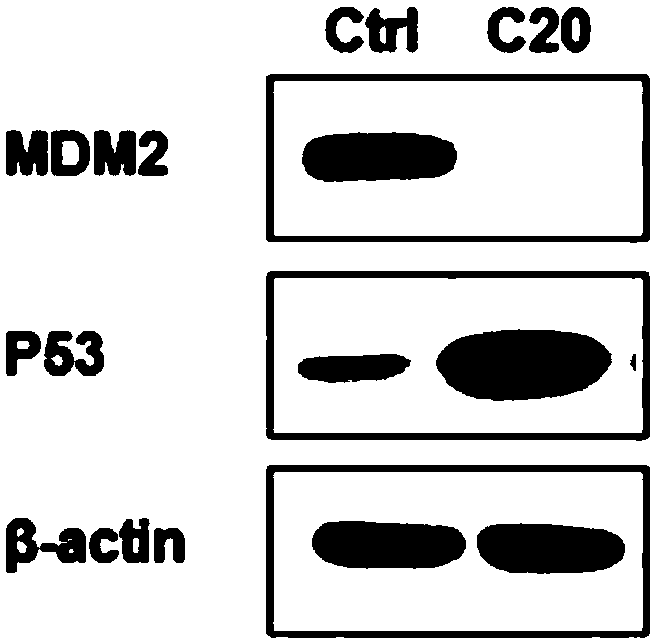
[0174] The results of the WesternBolt experiment are as follows: image 3 shown, by image 3 The experimental results showed that after the compound 15a was intervened in A549 cells at a concentration of 5 μM for 12 hours, the p53 protein began to be up-regulated and MDM2 began to be weakened. After the compound 15a was intervened at a concentration of 20 μM for 12 hours, the p53 protein was significantly up-regulated and the MDM2 protein was significantly weakened.
[0175]At ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com