Haloacetic acid-containing chitosan quaternary ammonium salt as well as preparation method and application thereof
A technology of chitosan quaternary ammonium salt and halogenated acetic acid, which is applied in the field of marine chemical engineering, can solve problems such as application limitations, and achieve the effects of low material cost, high yield, and improved antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
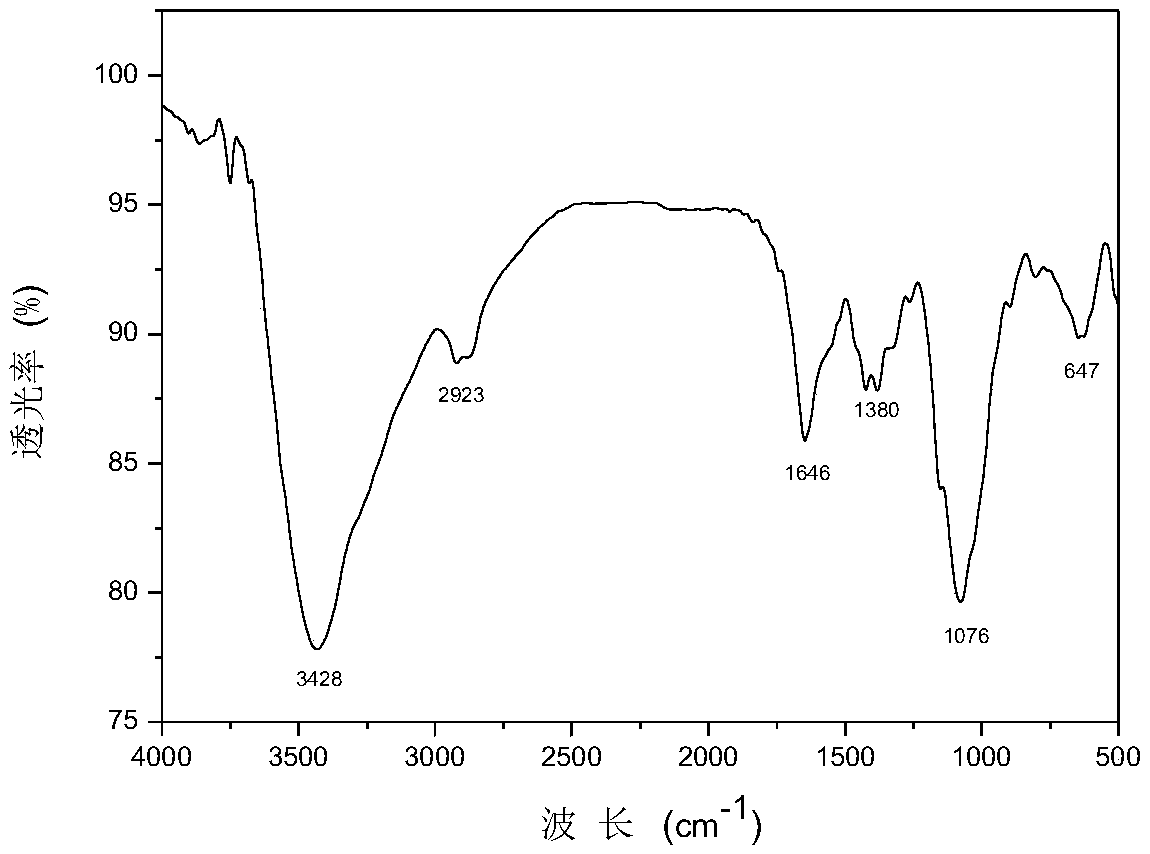
[0032] The structural formula of chitosan quaternary ammonium salt containing chloroacetic acid is as formula (1), wherein the average value range of n is 300-900.
[0033] Preparation of chitosan quaternary ammonium salt: using chitosan, 3-chloro-2-hydroxypropyltrimethylammonium chloride, sodium hydroxide, isopropanol and halogenated acetic acid compounds as raw materials, take 1g chitosan Disperse the sugar in 20mL of isopropanol and swell, stir at room temperature for 4 hours, add 6mL of 3-chloro-2-hydroxypropyltrimethylammonium chloride and 1g of sodium hydroxide solution in sequence, and continue the reaction at 75°C for 12 hours. Then use appropriate amount of ethanol to precipitate, wash and dry to obtain chitosan quaternary ammonium salt.
[0034] Dissolve 1 g of the chitosan quaternary ammonium salt in water, dialyze in 100 mL of 3% sodium chloroacetate solution for 12 hours, then remove the sodium haloacetate solution, dialyze in distilled water for 24 ho...
Embodiment 2
[0038]
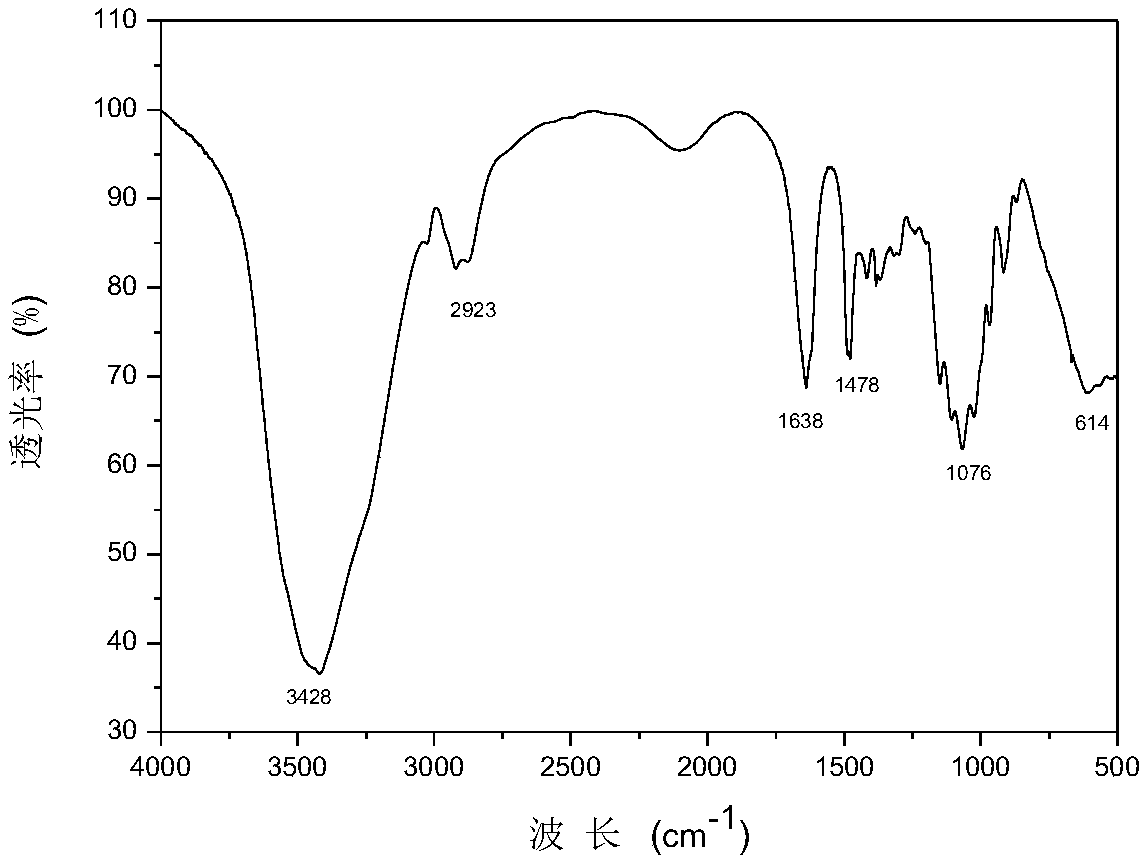
[0039] The structural formula of chitosan quaternary ammonium salt containing dichloroacetic acid is as formula (2), wherein the average value range of n is 300-900.
[0040] The difference from Example 1 is:
[0041] Using chitosan, 3-chloro-2-hydroxypropyltrimethylammonium chloride, sodium hydroxide, isopropanol and halogenated acetic acid compounds as raw materials, take 1.2g chitosan and disperse in 20mL isopropanol Swell, stir at room temperature for 4 hours, add 3-chloro-2-hydroxypropyltrimethylammonium chloride 8mL, sodium hydroxide solution 1.2g, continue to react for 12 hours, then precipitate and wash with an appropriate amount of ethanol to obtain chitosan Sugar quaternary ammonium salt.
[0042] 1 g of the chitosan quaternary ammonium salt was dissolved in 20 mL of distilled water, slowly added dropwise to 15% sodium dichloroacetate solution, then the solution was dialyzed in distilled water for 24 hours, concentrated and then freeze-dried to obtain ch...
Embodiment 3
[0045]
[0046] The structural formula of chitosan quaternary ammonium salt containing trichloroacetic acid is as formula (3), wherein the average value range of n is 300-900.
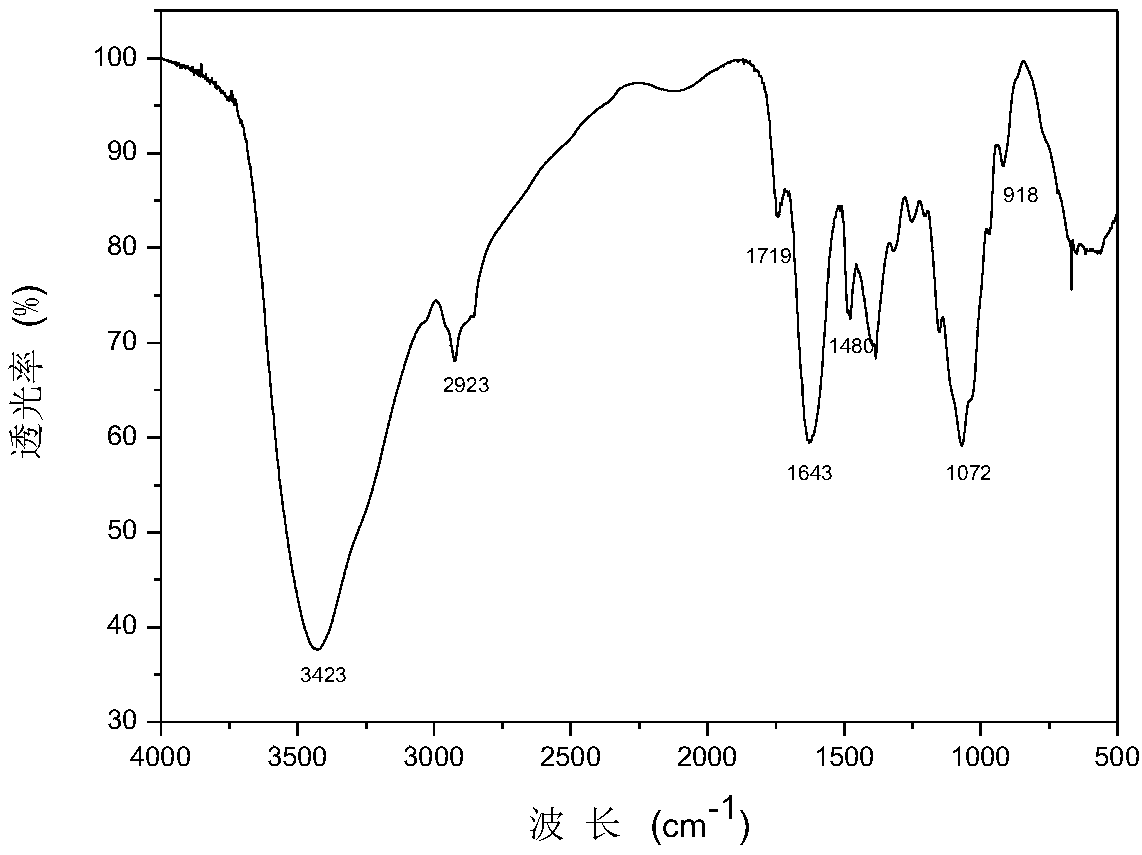
[0047] The difference from Example 1 is:
[0048] Using chitosan, 3-chloro-2-hydroxypropyltrimethylammonium chloride, sodium hydroxide, isopropanol and halogenated acetic acid compounds as raw materials, take 1.5g chitosan and disperse in 20mL isopropanol Swell, stir at room temperature for 4 hours, add 10 mL of 3-chloro-2-hydroxypropyltrimethylammonium chloride, 6 mL of 40% sodium hydroxide solution in turn, continue to react for 12 hours, then precipitate and wash with an appropriate amount of ethanol to obtain Chitosan quaternary ammonium salt.
[0049]The chitosan quaternary ammonium salt 1.3g was dissolved in 20mL distilled water, slowly added dropwise to 15% sodium trichloroacetate solution, then the solution was dialyzed in distilled water for 24h, concentrated and freeze-dried to obtain a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com