Noble metal catalyst and preparation method and application thereof
A noble metal catalyst and noble metal technology, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as limited catalytic activity and complicated preparation process, and achieve increased Effects of activity, high catalytic activity and sintering resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve 100mg KBr in 20ml deionized water, then heat the solution to 60°C for tens of minutes to accelerate the dissolution, PdCl 2 And NaCl preparation concentration is 5×10 -4 mol / L Na 2 PdCl 4 aqueous solution, then mixed with 1 x 10 -3 mol / L of SnCl 2 The aqueous solution was sequentially added to the above KBr solution. The above mixed solution was heated at 60° C. for 1 hour. Centrifuge after cooling, wash the obtained solid twice with water and once with ethanol, and then dry it.
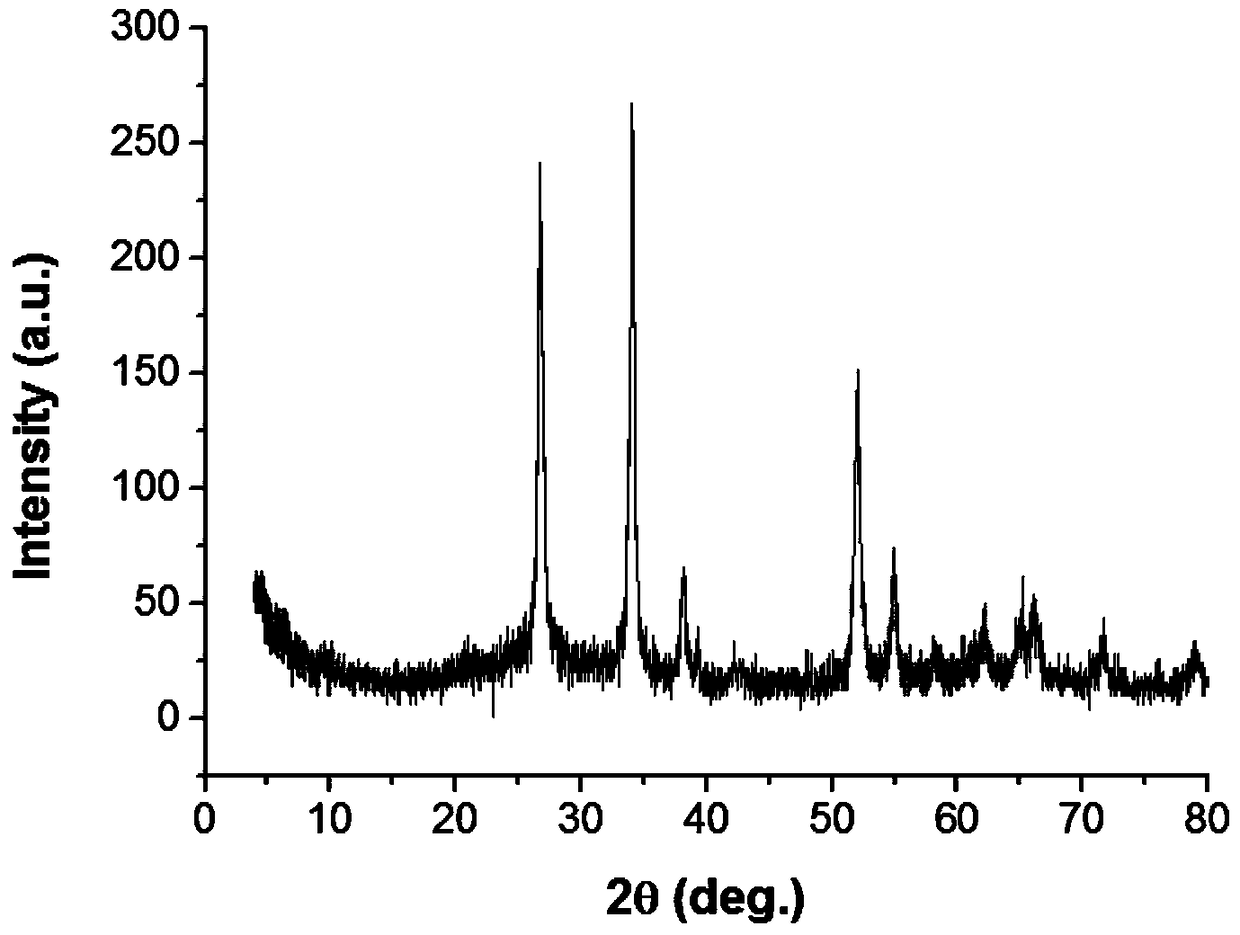
[0032] Carry out XRD and TEM test to the catalyst that present embodiment makes, its result is as follows figure 1 with figure 2 It can be seen from the lattice fringe changes observed by the transmission electron microscope that part of the noble metal has entered the various substituted divalent tin ions of tin dioxide. EDS analysis was carried out, and the results are shown in Table 1. Among them, the Cu and Si elements come from the self-contained elements of the instrume...
Embodiment 2
[0036] 100 mg of KBr was dissolved in 20 ml of deionized water, and the solution was heated to 60 °C for tens of minutes. Using PdCl 2 And NaCl preparation concentration is 5×10 -5 mol / L Na 2 PdCl 4 aqueous solution, then mixed with 1 x 10 -4 mol / L SnCl 2The aqueous solution was sequentially added to the above KBr solution. The above mixed solution was heated at 60° C. for 1 hour. Centrifuge after cooling, wash the obtained solid twice with water and once with ethanol, and then dry it.
Embodiment 3
[0038] Dissolve 50mg KBr in 20ml deionized water, add 5×10 -3 K in mol / L 2 PtCl 4 aqueous solution, then mixed with 1 x 10 -2 mol / L of SnCl 2 The aqueous solution was sequentially added to the above KBr solution. The above mixed solution was heated at 100°C for 0.5 hours. Centrifuge after cooling, wash the obtained solid twice with water and once with ethanol, and then dry it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com