Salen metal complex catalyst, as well as preparation method and application thereof
A metal complex and catalyst technology, applied in the field of salen metal complex catalyst and preparation thereof, can solve the problems of low yield of methyl lactate, cannot be reused, long preparation time and the like, achieves short preparation time, simple preparation method, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
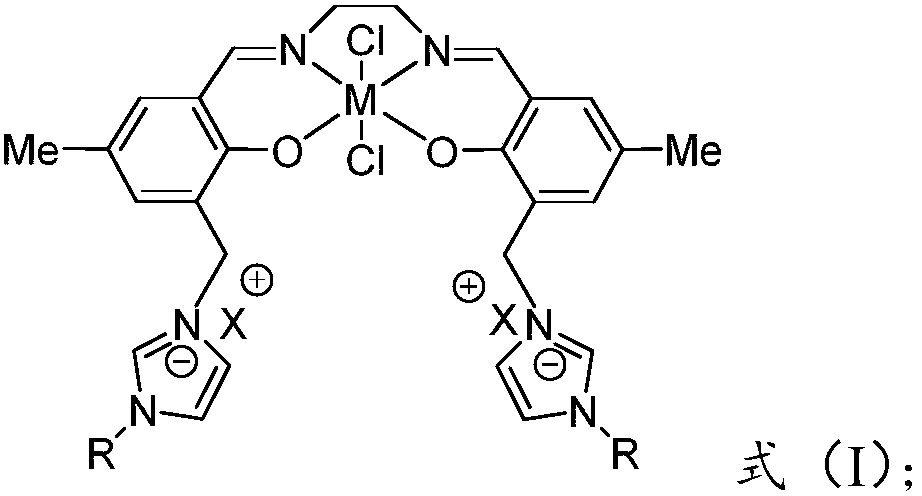
[0053] Prepare salen-x (x=Al, Zn, Sn) catalyst, and its preparation steps are as follows:
[0054] 1. Preparation of salen ligand:
[0055] Add 50 mL of ethylenediamine (10 mmol) in absolute ethanol solution dropwise to 100 mL of salicylaldehyde (10 mmol) ligand in absolute ethanol solution, and react under reflux at 40° C. for 12 h. After the reaction, the solvent was evaporated by rotary evaporation to obtain a light yellow viscous functional salen ligand, which was dried under vacuum at 40° C. overnight.
[0056] 2. Preparation of salen-x (x=Al, Zn, Sn) metal complex catalyst:
[0057] Dissolve the salen ligand (5mmol) prepared in step 1 in 50mL of anhydrous chloroform, slowly drop into 100mL of anhydrous chloroform solution containing metal compounds (dichloroethylaluminum, zinc acetate, tin chloride), at 60°C Under continuous stirring and reflux reaction for 24h. After the reaction, the solvent was evaporated by rotary evaporation, washed several times with anhydrous e...
Embodiment 2
[0059] Prepare polyether type ionic liquid functional metal complex salen-x-[OMIm]Br (x=Al, Zn, Sn) catalyst, its preparation steps are as follows:
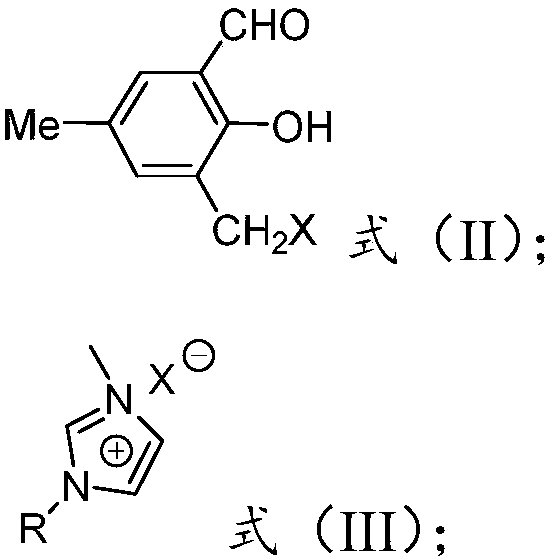
[0060] 1. Synthesis of 1.3-bromomethyl-5-methyl salicylaldehyde:
[0061] Add 5-methylsalicylaldehyde (10mmol), paraformaldehyde (10mmol), 40% HBr (15mL) and a small amount of concentrated sulfuric acid in a 500mL round bottom flask in sequence, and the mixture was magnetically stirred at 80°C for 12h, then cooled to room temperature , add 20 mL of water and 40 mL of CH to the mixture 2 Cl 2 For extraction, use anhydrous Na 2 SO 4 The organic phase is dried. CH removal by distillation under reduced pressure 2 Cl 2 , to obtain 3-bromomethyl-5-methylsalicylaldehyde as a white solid.
[0062] 2. Synthesis of modified salicylaldehyde:
[0063] Under nitrogen protection, 1-butyl-3-methylimidazolium bromide (10mmol) was dissolved in 100mL of anhydrous toluene, and 3-bromomethyl-5-methylsalicylaldehyde (10mmol) was added dropwis...
Embodiment 3
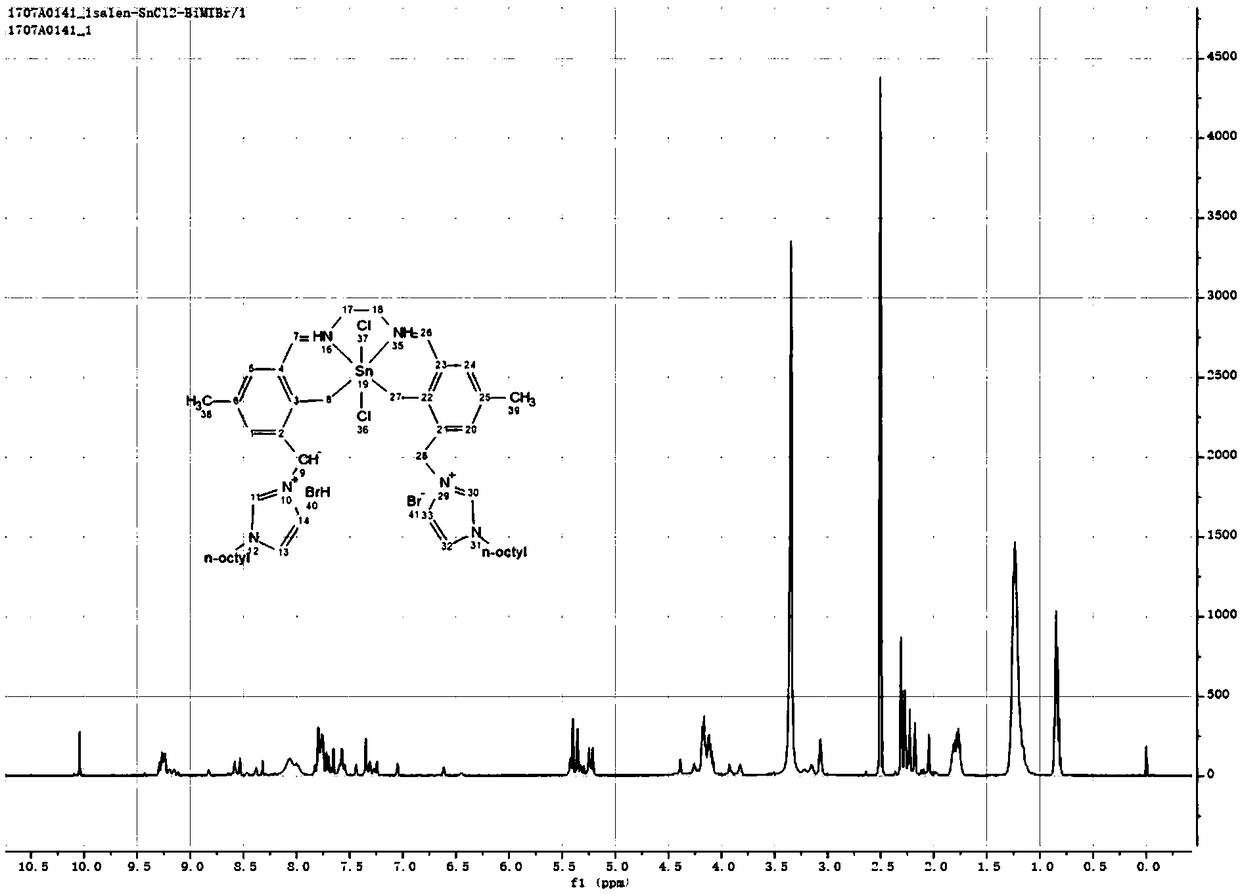
[0067] Using a series of salen-x (x=Al, Zn, Sn) metal complex catalysts prepared in Example 1 to catalyze methyl lactate from fructose:
[0068] Weigh 0.3g fructose, 0.05g salen-x (x=Al, Zn, Sn) metal complex catalyst, 12g methanol and 25mL stainless steel autoclave, feed 2MPa nitrogen at room temperature and slowly discharge it, repeat 3 Finally, feed 2MPa nitrogen gas, react at 160°C for 2 hours under the stirring condition of 600rpm, and centrifuge the catalyst after the reaction is completed. Determination of the productive rate of methyl lactate and related by-products, the results are shown in Table 1.
[0069] From the results in Table 1, it can be known that the salen-Sn catalytic conversion of fructose prepared in Example 1 of the present invention has the highest yield of methyl lactate, and the yield of methyl lactate is 36%.
[0070] Table 1 The conversion rate of fructose and the yield of methyl lactate and related by-products under different catalysts
[0071] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com