Triazole-based compound and preparation method thereof
A technology of triazoles and compounds, which is applied in the field of preparation of drugs against wheat sheath blight pathogens, and achieves the effects of simple and easy reaction operation, large profit margin and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
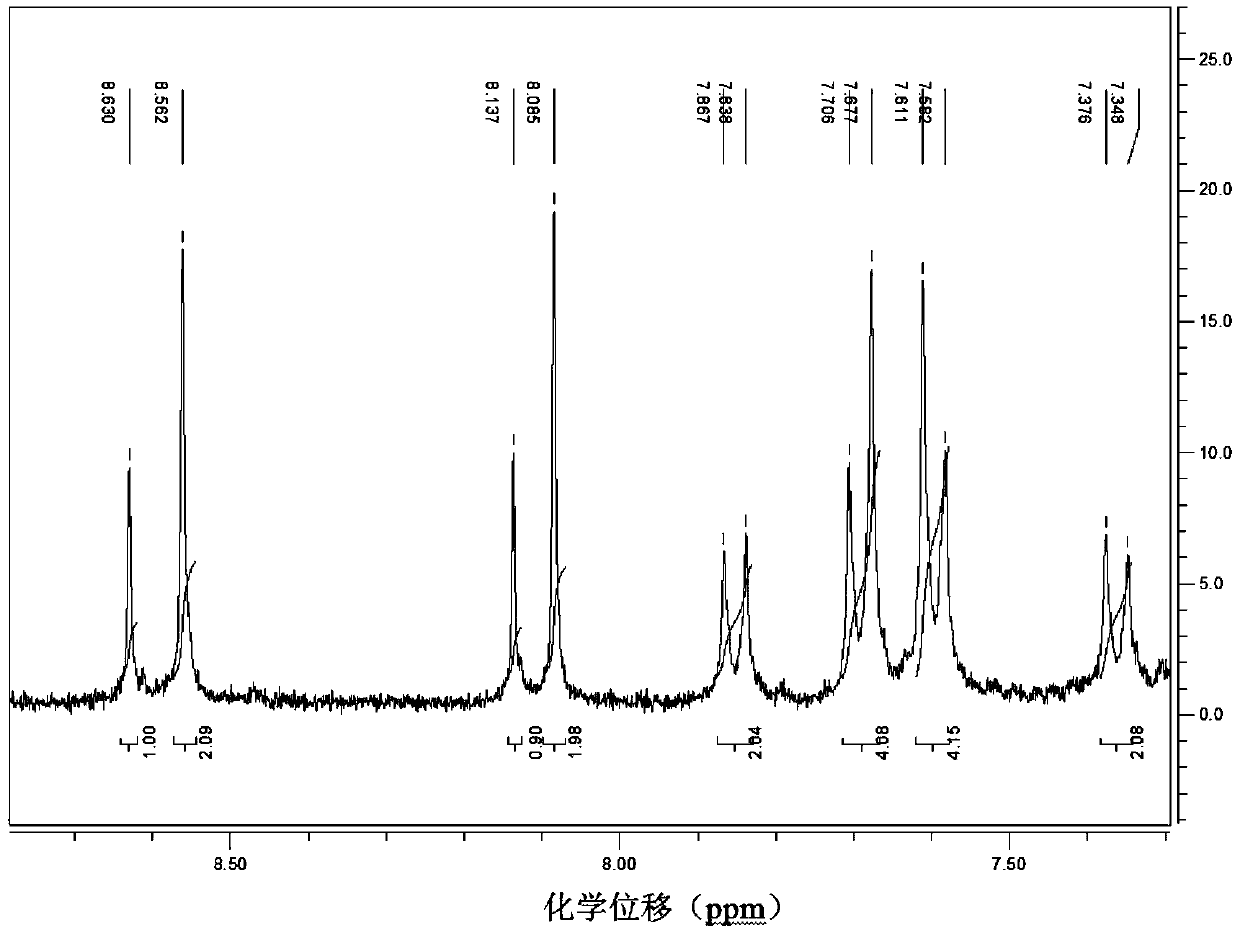
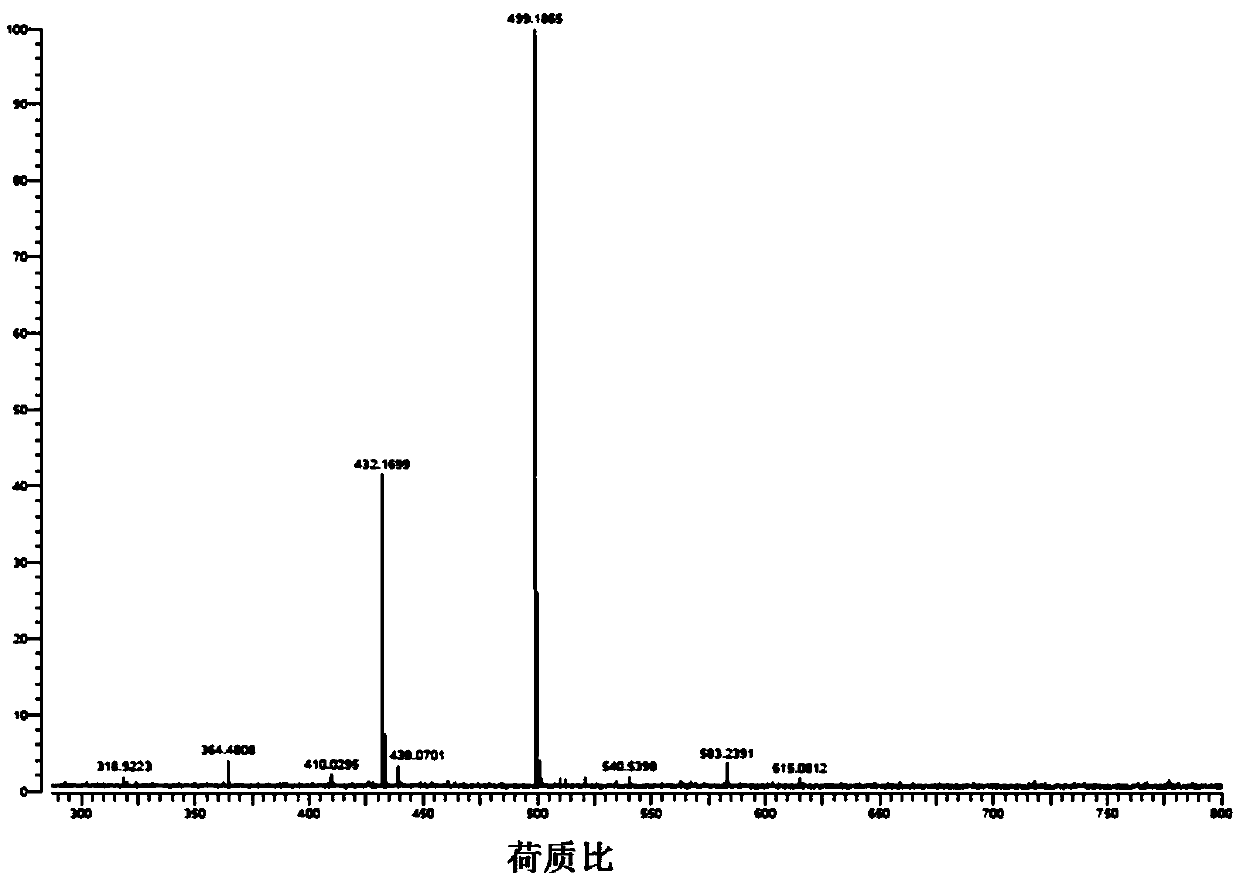
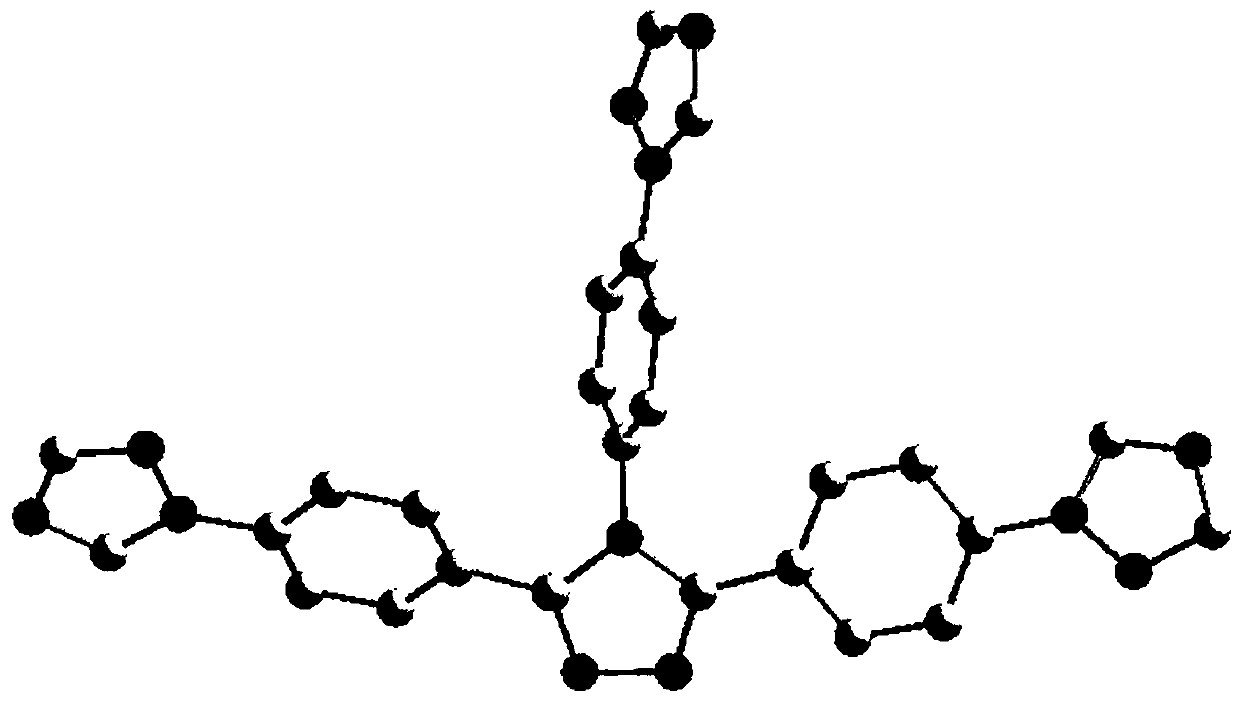
[0030] Add CuO (0.0398g, 0.5mmol), potassium carbonate (0.691g, 5mmol), 1,2,4-triazole (0.518g , 7.5mmol), 3,4,5-tris(4-bromophenyl)-1H-1,2,4-triazole (0.498g, 1mmol), 20mL DMF. Start stirring and react at 150° C. for 48 hours. After the reaction, the reaction solution was lowered to room temperature at 20-25 degrees Celsius, filtered, and 50 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered with suction, the filter cake was collected, and recrystallized with ethanol to obtain 3,4,5-tris(4-(1H -1,2,4-triazol-1-yl)phenyl)-4H-1,2,4-triazole single crystal, yield 68.8%. Nuclear Magnetic Spectrum-Proton Spectrum, High-resolution Mass Spectrum, please refer to figure 1 , figure 2 .
[0031] It can be seen from the hydrogen spectrum 1 H NMR (400MHz, CDCl 3 )δ: 7.35-7.40 (d, 2H, Ar-H), 7.55-7.63 (d, 4H, Ar-H), 7.66-7.78 (d, 4H, Ar-H), 7.88-7.90 (d, 2H, Ar-H),8.05-8.10(s,2H,N=CH-N),8.12-8.15(s,1H,N=CH-N),8.50-8.65(s,2H,N=CH-N), 8....
Embodiment 2
[0036] Add CuO (0.0398g, 0.5mmol), potassium carbonate (0.691g, 2.5mmol), triazole (0.518g, 4.5mmol), 3,4,5-Tris(4-bromophenyl)-1H-1,2,4-triazole (0.498 g, 1 mmol), 20 mL DMF. Start stirring at 120°C and react for 36 hours. After the reaction, the reaction solution was cooled to room temperature, filtered, and 50 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered by suction, and the filter cake was collected, with a yield of 70%. Use the same test method to detect, showing the characteristic peaks that are basically the same as those in the nuclear magnetic spectrum and mass spectrum in Example 1, indicating that the products are consistent, and both are 3,4,5-tri(4-(1H-1,2,4-tri Azol-1-yl)phenyl)-4H-1,2,4-triazole.
Embodiment 3
[0038] Antifungal activity of 3,4,5-tris(4-(1H-1,2,4-triazol-1-yl)phenyl)-4H-1,2,4-triazole of the present invention experiment.
[0039] Main Experimental Instruments
[0040]
[0041] main experimental materials
[0042] a. Drug to be tested: 3,4,5-tris(4-(1H-1,2,4-triazol-1-yl)phenyl)-4H-1,2,4-triazol prepared by the present invention azole.
[0043] b. Medium: potato medium (potato, agar, sucrose)
[0044] c. Species to be tested: pathogenic bacteria of wheat sheath blight (the pathogenic bacteria were collected from plant samples where the pathogenic bacteria occurred in Xiqing District, Tianjin, and separated and collected in July 2015).
[0045] d. Solvent: distilled water, DMSO
[0046] The "inhibition zone method" is used for testing, which can also be called the "colony growth diameter method" (Yang Zhihui, Zhu Jiehua, Guo Qiang, Zhang Zhiming. Preliminary study on the biological characteristics of single spore isolates of potato infestans [J]. . Mycosystem,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com