A kind of flupenthixol derivative and preparation method thereof
A technology of flupenthixol and synthetic method, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
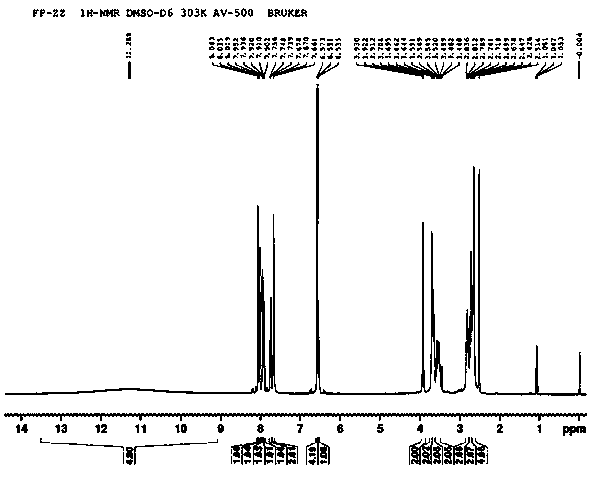
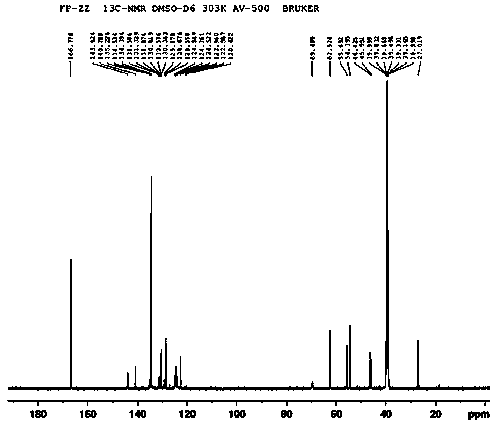
Image
Examples
Embodiment 1
[0030] Preparation of compound 9-(1-hydroxy-3-(4-(2-hydroxyethyl)piperazin-1-yl)propyl)-2-(trifluoromethyl)-9H-sulfanthene fumarate
[0031] Weigh 217.08mg (0.5mmol) of flupenthixol into a round bottom flask, dissolve it with 30mL of acetone, add 1.27mg (0.005mmol) of osmium tetroxide and 10.43mg (0.045mmol) of silver oxide, and stir thoroughly at 5°C After 3 hours of reaction, the catalyst was removed from the reaction solution with mercapto silica gel to obtain intermediate 1, which weighed 156.44 mg (about 0.336 mmol, and the yield was 67.12%; As for the malic acid in the reactor, add 20 mL of ethanol, stir at room temperature for 0.5 h, evaporate the ethanol under reduced pressure, add 30 mL of acetone to the residue, heat to dissolve, decolorize with activated carbon for 0.5 h, filter, add 20 mL of ether to the filtrate, Stir vigorously under ice-water cooling for 3 hours, crystals precipitate out, filter, wash with a mixed solution (acetone: ether = 3:2), and dry in vacu...
Embodiment 2
[0033] Preparation of compound 9-(1-hydroxy-3-(4-(2-hydroxyethyl)piperazin-1-yl)propyl)-2-(trifluoromethyl)-9H-sulfanthene fumarate
[0034] Weigh 217.08mg (0.5mmol) of flupenthixol into a round bottom flask, dissolve it with 30mL of DMSO, add 1.27mg (0.005mmol) of osmium tetroxide and 10.43mg (0.045mmol) of silver oxide, and fully stir the reaction at 0°C After 4 h, the catalyst was removed from the reaction solution with mercapto silica gel to obtain intermediate 1, which weighed 140.20 mg (about 0.300 mmol, and the yield was 60.15%; the reaction intermediate 1 and 69.61 mg (about 0.600 mmol) As for the malic acid in the reactor, add 20 mL of ethanol, stir at room temperature for 0.5 h, evaporate the ethanol under reduced pressure, add 30 mL of acetone to the residue, heat to dissolve, decolorize with activated carbon for 0.5 h, filter, add 20 mL of ether to the filtrate, Stir vigorously under ice-water cooling for 3 hours, crystals precipitate out, filter, wash with a mixed...
Embodiment 3
[0036] Preparation of compound 9-(1-hydroxy-3-(4-(2-hydroxyethyl)piperazin-1-yl)propyl)-2-(trifluoromethyl)-9H-sulfanthene fumarate
[0037] Weigh 217.08mg (0.5mmol) of flupenthixol into a round bottom flask, dissolve it with 30mL of acetone, add 1.91mg (0.0075mmol) of osmium tetroxide and 11.59mg (0.05mmol) of silver oxide, and stir thoroughly at 5°C After 3 hours of reaction, the catalyst was removed from the reaction solution with mercapto silica gel to obtain intermediate 1, which weighed 147.30 mg (about 0.316 mmol, and the yield was 63.12%; As for the malic acid in the reactor, add 20 mL of ethanol, stir at room temperature for 0.5 h, evaporate the ethanol under reduced pressure, add 30 mL of acetone to the residue, heat to dissolve, decolorize with activated carbon for 0.5 h, filter, add 20 mL of ether to the filtrate, Stir vigorously under ice-water cooling for 3 hours, crystals precipitate out, filter, wash with a mixed solution (acetone: ether = 3:2), and dry in vacu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com