Ginsenoside composition with hypoglycemic activity and application thereof
A technology of ginsenosides and compositions, applied in the field of ginsenosides compositions, to achieve the effects of improving the hypoglycemic effect, reducing costs, and facilitating drug development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
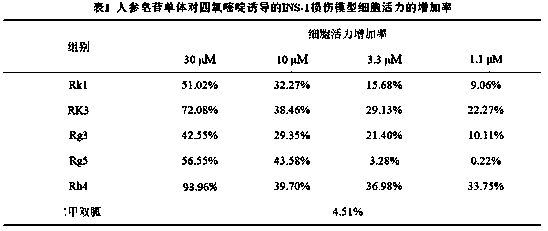
[0040] Example 1 In vitro hypoglycemic experiment of ginsenoside monomers
[0041] Experimental drug: ginsenosides in diol group: ginsenosides Rk1, Rg3, Rg5;
[0042] Triol group ginsenosides: ginsenosides Rk3, Rh4;
[0043] The purity of the above drugs were >98%.
[0044] Experimental cells: INS-1 rat insulinoma cells, HepG2 human liver cancer cells and 3T3-L1 mouse preadipocytes were all purchased from Shanghai Bogu Biotechnology Co., Ltd., and the generations were within 10 generations.
[0045] Test method and result:
[0046] 1. Effect of ginsenoside monomers on the viability of alloxan-induced INS-1 injury model cells
[0047] After recovery, the INS-1 cells were transferred to a 100ml culture flask with RPMI 1640 culture medium containing 10% inactivated calf serum, and incubated at 37°C and 5% CO 2 cultivated under conditions. When the cells adhered to the wall and overgrown, the medium was poured out, digested with 0.25% trypsin, and passaged once every 3 days a...
Embodiment 2
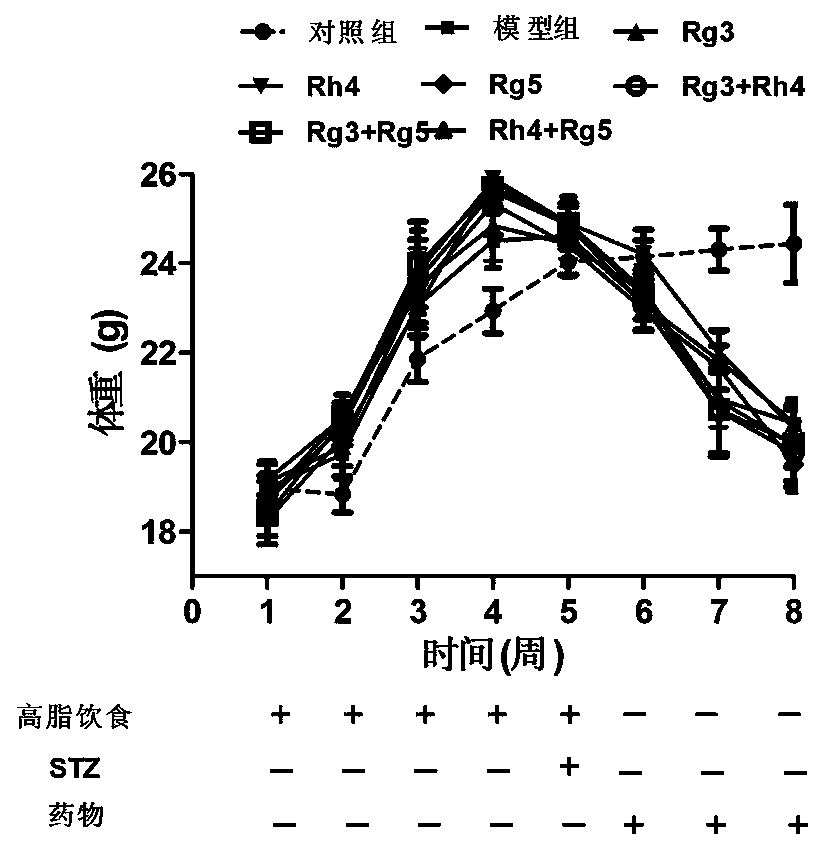
[0064] Example 2 The therapeutic effect of ginsenoside monomer and drug combination on type Ⅱ diabetes mouse model
[0065] Modeling: 110 healthy male, clean C57BL / 6 mice aged 5-6 weeks (body weight 18±2 g) were purchased from the Animal Center of Xi’an Jiaotong University School of Medicine. The mice were adaptively fed for 7 days and randomly divided into two groups, one group of 10 mice was given basic feed; the other group of 100 mice was given high-fat and high-sugar feed. After 6 weeks, the high-fat and high-sugar diet group was fasted for 12 hours, and then intraperitoneally injected STZ (prepared in citrate buffer) 30 mg / kg, once a day, for 5 consecutive days to establish a diabetes model. The mice were fasted (without water) for 12 hours, and 0.2 mL of blood was collected from the fundus venous plexus of each mouse, and the serum was separated. Take 10 μL of serum in the sample tube to measure the fasting blood glucose value of the mouse according to the operation me...
Embodiment 3
[0076] Example 3 Preparation of Ginsenoside Tablets
[0077] Take 90 g of ginsenoside composition (60 g of ginsenoside Rg5 and 30 g of ginsenoside Rh4), 70 g of sodium carboxymethyl cellulose, 200 g of microcrystalline cellulose, 70 g of sodium carboxymethyl starch, and 2 g of magnesium stearate. raw material.
[0078] Pass the above-mentioned main ingredients and auxiliary materials through 80-mesh sieve respectively, mix well, use 80% ethanol as binder, granulate with 16-mesh sieve, dry at 55-60°C, granulate with 14-mesh sieve, and press into tablets, each Tablet 0.4 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com