Preparation method and application of tin tetrachloride
A compound and polymer technology, applied in the preparation of organic compounds, tin chloride, chemical instruments and methods, etc., can solve the problems of chlorine gas channel blockage, short residence time, and large environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
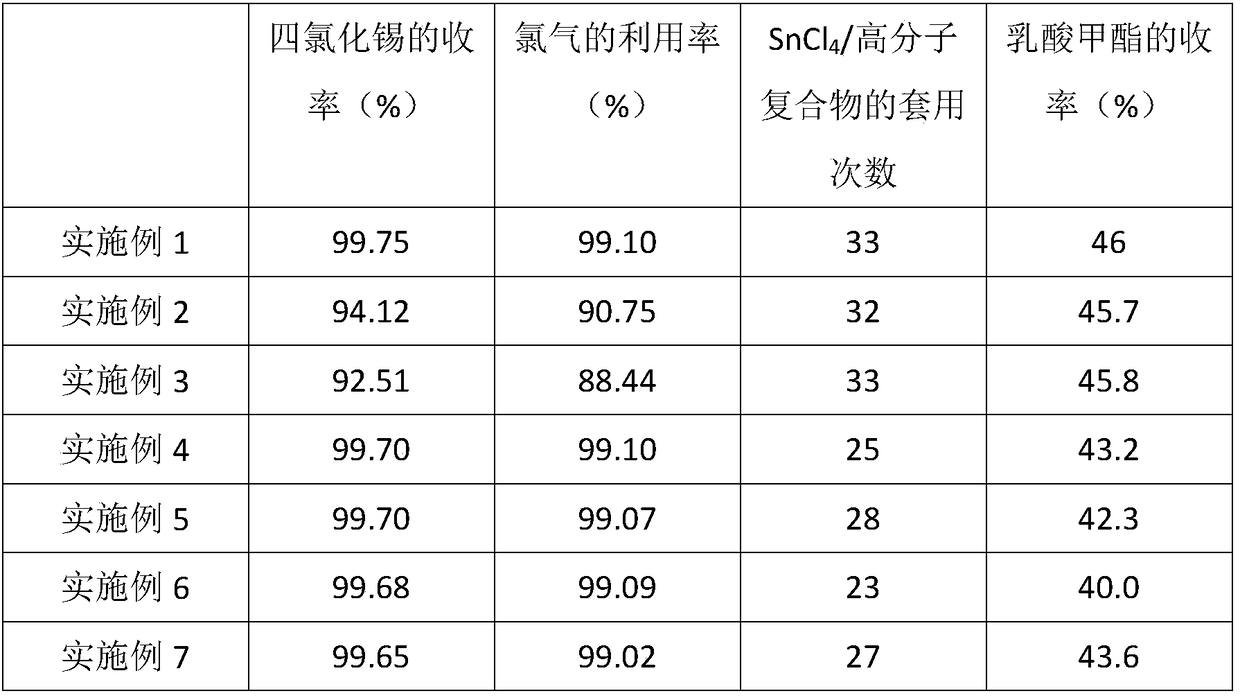
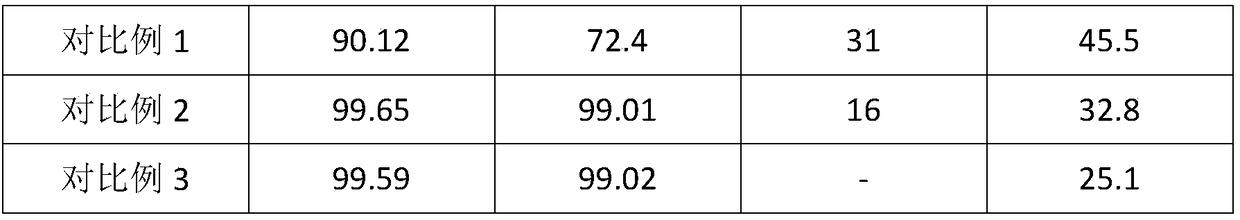
Examples
preparation example Construction
[0029] The first aspect of the present invention provides a kind of preparation method of tin tetrachloride, comprises the following steps:
[0030] Add tin, tin tetrachloride solution, and mesoporous silica to the reaction kettle, and then introduce chlorine gas, and control the temperature in the kettle to be lower than 100°C, control the pressure at 0.5MPa, and control the flow rate of chlorine gas at 31m 3 / h, reaction 16h; Get tin tetrachloride;
[0031] Wherein, the mesoporous silica is SBA-15 mesoporous silica; the weight ratio of the tin to the tin tetrachloride solution and the mesoporous silica is 1: (0.5-1.5): (0.2-0.7).
[0032] In one embodiment, the weight ratio of the tin to the tin tetrachloride solution and the mesoporous silica is 1:0.8:0.4.
[0033] In the present invention, because the reaction of tin and chlorine is an exothermic reaction and the solubility of chlorine in tin tetrachloride is large, using tin tetrachloride as the reaction medium not only...
Embodiment 1
[0052] The preparation method of the tin tetrachloride is to add tin, tin tetrachloride solution, and mesoporous silica into the reaction kettle, and then feed chlorine gas, and control the temperature in the kettle to be lower than 100°C, and the pressure to be controlled at 0.5MPa , the flow of chlorine gas is controlled at 31m 3 / h, reaction 16h; Get tin tetrachloride;
[0053] Wherein, the mesoporous silica is SBA-15 mesoporous silica; the weight ratio of the tin to the tin tetrachloride solution and the mesoporous silica is 1:0.8:0.4;
[0054] The SnCl 4 / The preparation method of macromolecule compound, comprises the following steps:
[0055] (1) benzoxazine monomer: melamine, octa(aminophenyltrioxysilane), phenol, and methanol / water solution with a mass fraction of 50% are added to the reactor in molar ratio, stirred, and heated to 55° C., Add formaldehyde solution dropwise, after the dropwise addition, raise the temperature to 85°C, keep the temperature for 7 hours,...
Embodiment 2
[0061] The preparation method of the tin tetrachloride is to add tin, tin tetrachloride solution, and nano silicon dioxide into the reaction kettle, and then feed chlorine gas, and control the temperature in the kettle to be lower than 100°C, and the pressure to be controlled at 0.5MPa, Chlorine gas flow is controlled at 31m 3 / h, reaction 16h; Get tin tetrachloride;
[0062] Wherein, the nano-silica is purchased from Hangzhou Wanjing New Material Co., Ltd.; the weight ratio of the tin to the tin tetrachloride solution and the nano-silica is 1:0.8:0.4;
[0063] The SnCl 4 / The preparation method of macromolecule compound, comprises the following steps:
[0064] (1) benzoxazine monomer: melamine, octa(aminophenyltrioxysilane), phenol, and methanol / water solution with a mass fraction of 50% are added to the reactor in molar ratio, stirred, and heated to 55° C., Add formaldehyde solution dropwise, after the dropwise addition, raise the temperature to 85°C, keep the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com