Application of mesenchymal stem cell culture from human umbilical cord or supernatant of mesenchymal stem cell culture
A technology of mesenchymal stem cells and cultures, applied in the field of mesenchymal stem cells, can solve the problem of high treatment costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 MSC Separation and Identification of Umbilical Cord Origin
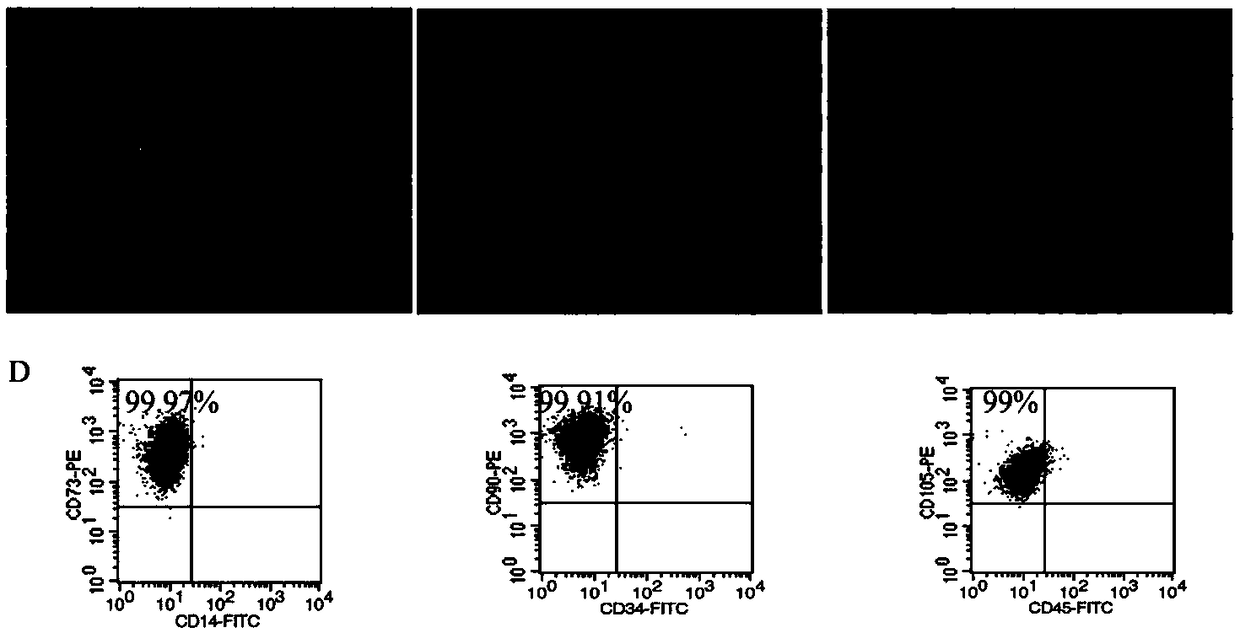
[0050] Take the umbilical cord from normal delivery, cut the umbilical cord into 1cm pieces in a sterile environment, put the cut umbilical cord tissue into α-MEM complete medium containing 10% FBS and culture it for 72 hours, and replace the culture medium every 2-3 days , when the crawled cells reached 80% confluency, add 0.25% trypsin to digest and passage. The primary human umbilical cord-derived MSCs were cultured for 5 days, and observed under a microscope, fibrous and adherent MSCs crawled out around the umbilical cord tissue. With the subculture MSC grows faster and the purity becomes higher, and all the cells grow in the shape of a long spindle and vortex adherent, which is consistent with the characteristics of MSC culture in vitro, such as figure 1 (A) shown. Collection of P3 generation MSCs showed high expression of CD105, CD73, CD166, etc., low expression or no expression of CD34, CD45...
Embodiment 2
[0051] Example 2 Growth Factors Highly Expressed by MSCs
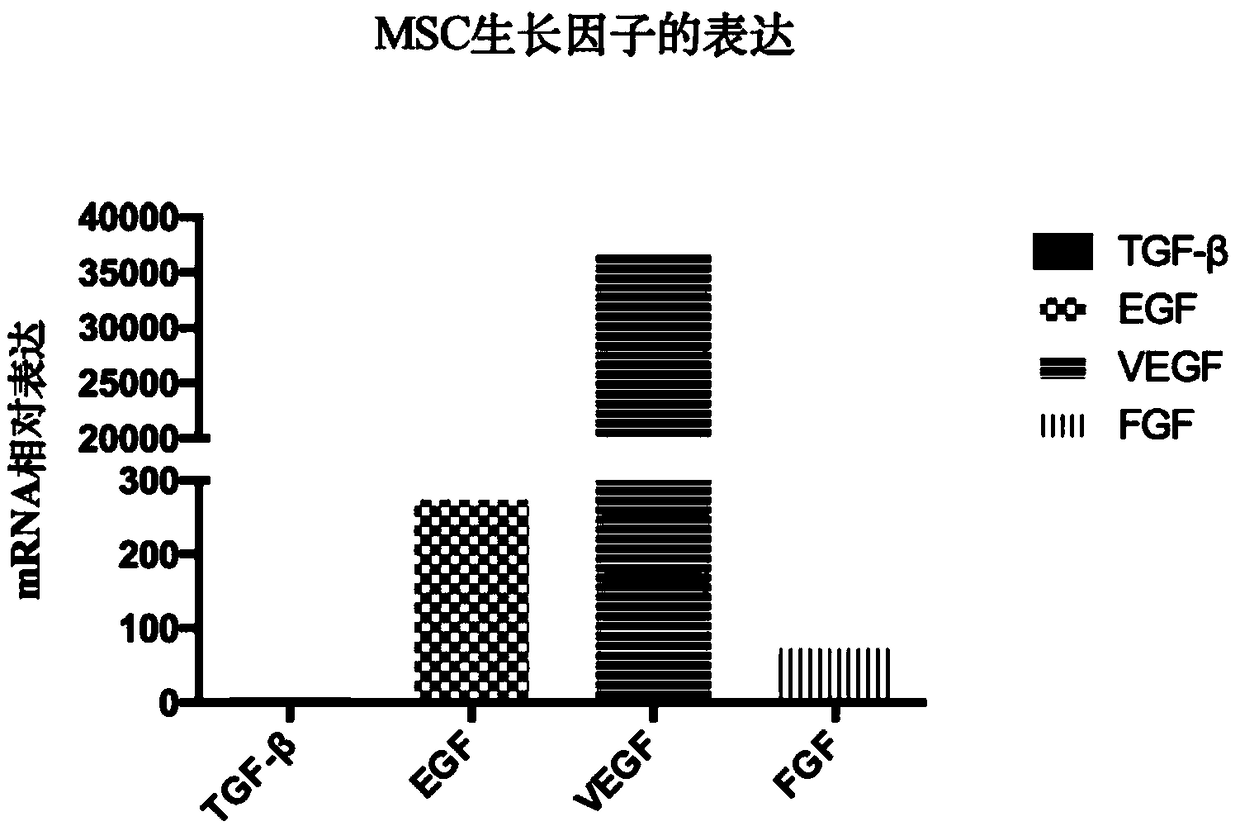
[0052] The growth and metabolism of MSC cells cultured in vitro will secrete some growth-promoting factors, so the expression of regenerative factors in MSC was detected. 0.25% trypsin digested and cultured MSCs of the P3 generation, extracted the total RNA of MSCs, and reverse-transcribed them into cDNA. The q-PCR results showed that MSCs could highly express TGF-β, EGF, FGF, VEGF and other related growth factors, and the expression level of VEGF highest, such as figure 2 shown.
Embodiment 3
[0053] The preparation of embodiment 3 culture or culture supernatant lyophilized powder
[0054] P3 generation MSCs were collected, cultured in serum-free medium for 48 hours, the serum-free MSC culture supernatant was aspirated, dead cells and debris were discarded by centrifugation, and the collected culture or culture supernatant was collected.
[0055] The collected collected culture or culture supernatant is desalted and decolorized by gel filtration, put into the freeze-drying chamber of the vacuum freeze-dryer, and carried out according to the procedures set by the biological characteristics of the protein, such as pre-cooling and freeze-drying. Preparation of dry powder, collecting the powder after the program runs. The freeze-drying procedure is as follows:
[0056] 1. Pre-freezing stage: -50℃, 2h;
[0057] 2. Initial evaporation stage: -28°C, 8h;
[0058] 3. Drying: 8°C, 6h;
[0059] 4. Freeze-drying: 27°C, 6h.
[0060] Weigh 30mg of powder and dissolve it in 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com