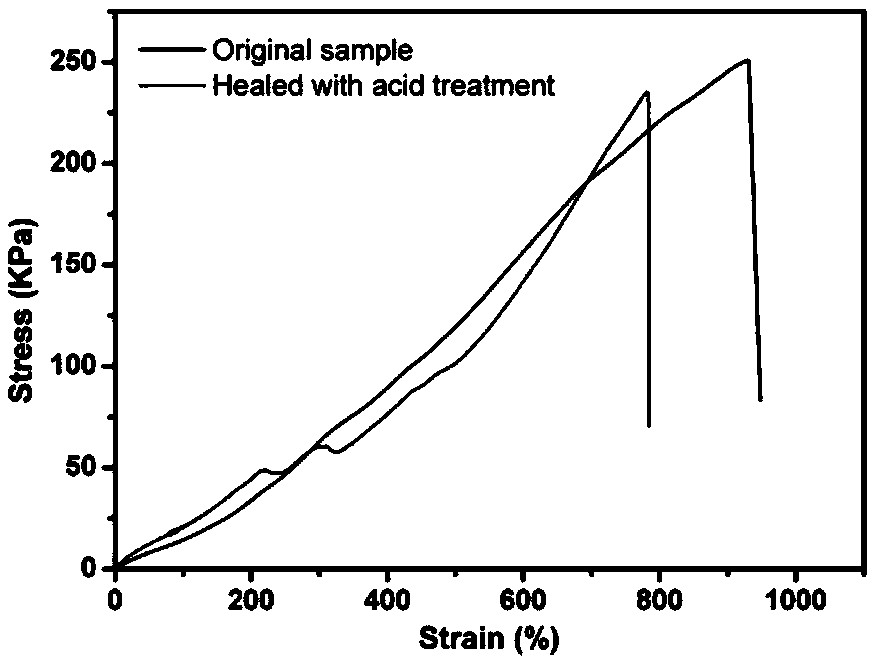
Dual-network hydrogel with high-toughness, shape-memory and self-repairing characteristics and preparation method of dual-network hydrogel
A dual-network and hydrogel technology, applied in the field of double-network hydrogel and its preparation, can solve the problems of poor mechanical properties and limit the application range of chitosan-based hydrogel, and achieve improved mechanical properties and good mechanical properties and self-healing performance, widening the effect of the application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
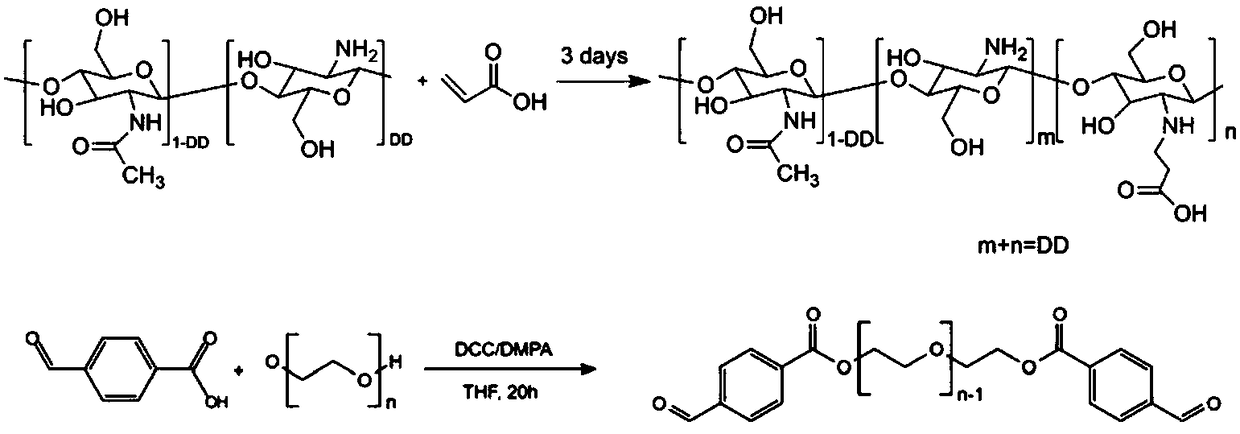
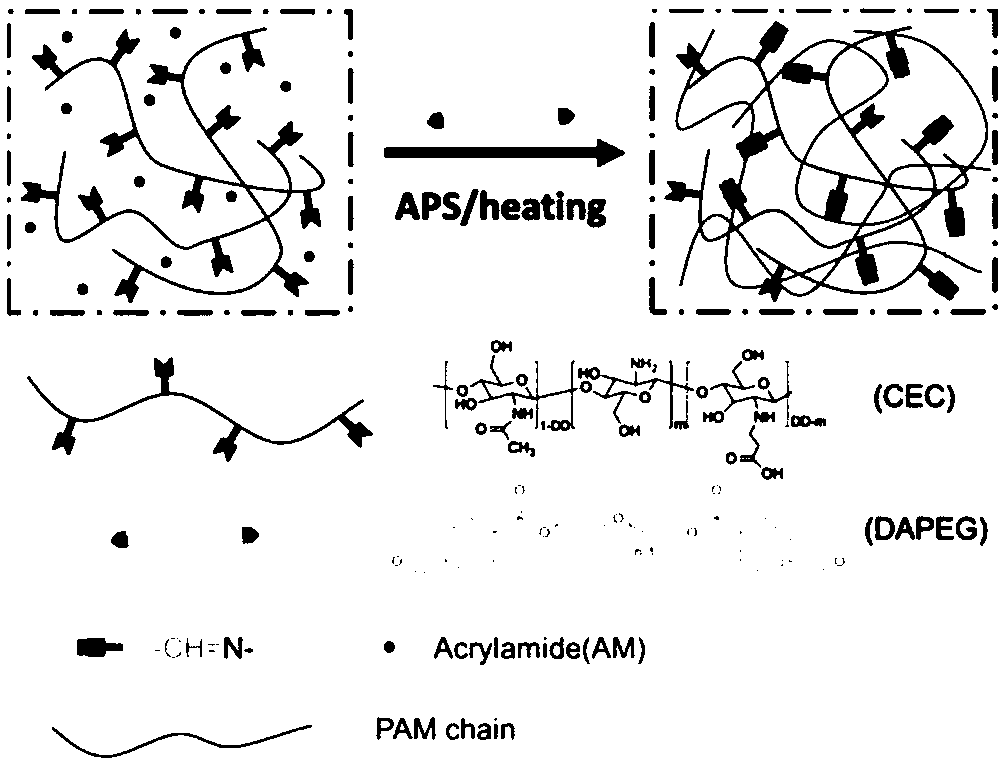
[0024] 1) Weigh 4g of chitosan with a viscosity-average molecular weight of 10w and a deacetylation degree of 85%, and disperse it in 200mL of deionized water, add 5.84mL (85.2mmol) of acrylic acid, stir and dissolve, and react at 50°C for 3 days. After the reaction, the pH of the product was adjusted to 10-12 with sodium hydroxide solution, then transferred to a dialysis bag for dialysis for 3 days, freeze-dried to obtain N-carboxyethyl chitosan (CEC);
[0025] 2) Dissolve 6.52g (1.63mmol) polyethylene glycol (PEG4000), 0.98g (6.52mmol) 4-formylbenzoic acid, 0.05g (0.407mmol) 4-(dimethylamino)pyridine (DMAP) in 200mL in anhydrous tetrahydrofuran. Under nitrogen atmosphere, 1.68g (8.15mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added, and reacted at 20°C for 18h. After the reaction, the white solid was filtered off to obtain a filtrate containing the product. The filtrate was precipitated with ether, and the filter cake obtained by filtration was dissolved in tetrahydro...
Embodiment 2
[0032] 1) Weigh 4g of chitosan with a viscosity-average molecular weight of 10w and a deacetylation degree of 95%, and disperse it in 200mL of deionized water, add 5.84mL (85.2mmol) of acrylic acid, stir and dissolve, and react at 50°C for 3 days. After the reaction, the pH of the product was adjusted to 10-12 with sodium hydroxide solution, then transferred to a dialysis bag for dialysis for 3 days, freeze-dried to obtain N-carboxyethyl chitosan (CEC);
[0033] 2) Dissolve 6.52g (1.63mmol) polyethylene glycol (PEG4000), 0.98g (6.52mmol) 4-formylbenzoic acid, 0.05g (0.407mmol) 4-(dimethylamino)pyridine (DMAP) in 200mL in anhydrous tetrahydrofuran. Under nitrogen atmosphere, 1.68g (8.15mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added, and reacted at 20°C for 18h. After the reaction, the white solid was filtered off to obtain a filtrate containing the product. The filtrate was precipitated with ether, and the filter cake obtained by filtration was dissolved in tetrahydro...
Embodiment 3
[0039] 1) Weigh 4g of chitosan with a viscosity-average molecular weight of 100w and a deacetylation degree of 50% and disperse it in 200mL of deionized water, add 5.84mL (85.2mmol) of acrylic acid and stir to dissolve, and react at 50°C for 3 days. After the reaction, the pH of the product was adjusted to 10-12 with sodium hydroxide solution, then transferred to a dialysis bag for dialysis for 3 days, freeze-dried to obtain N-carboxyethyl chitosan (CEC);
[0040] 2) Dissolve 3.27g (1.63mmol) polyethylene glycol (PEG2000), 0.98g (6.52mmol) 4-formylbenzoic acid, 0.05g (0.407mmol) 4-(dimethylamino)pyridine (DMAP) in 200mL in anhydrous tetrahydrofuran. Under nitrogen atmosphere, 1.68g (8.15mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added, and reacted at 20°C for 18h. After the reaction, the white solid was filtered off to obtain a filtrate containing the product. The filtrate was precipitated with ether, and the filter cake obtained by filtration was dissolved in tetrahyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com