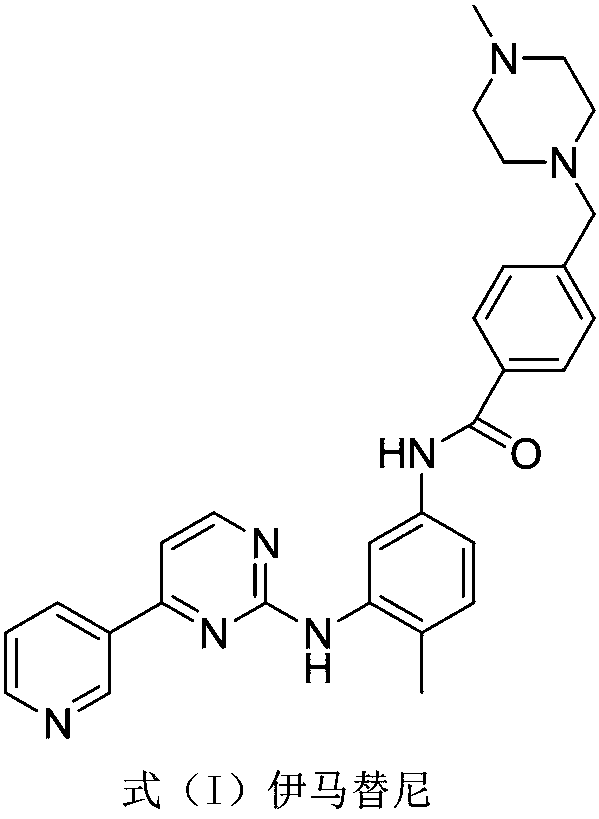
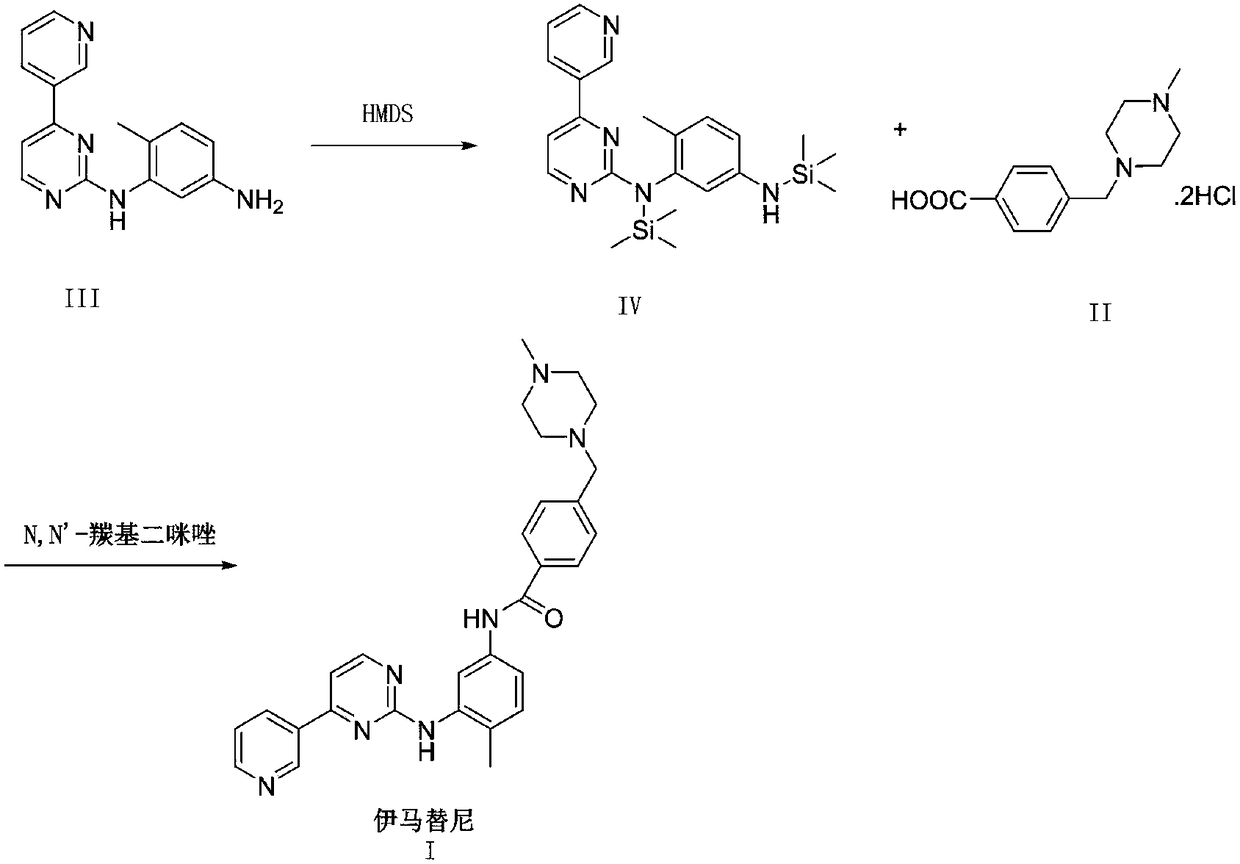
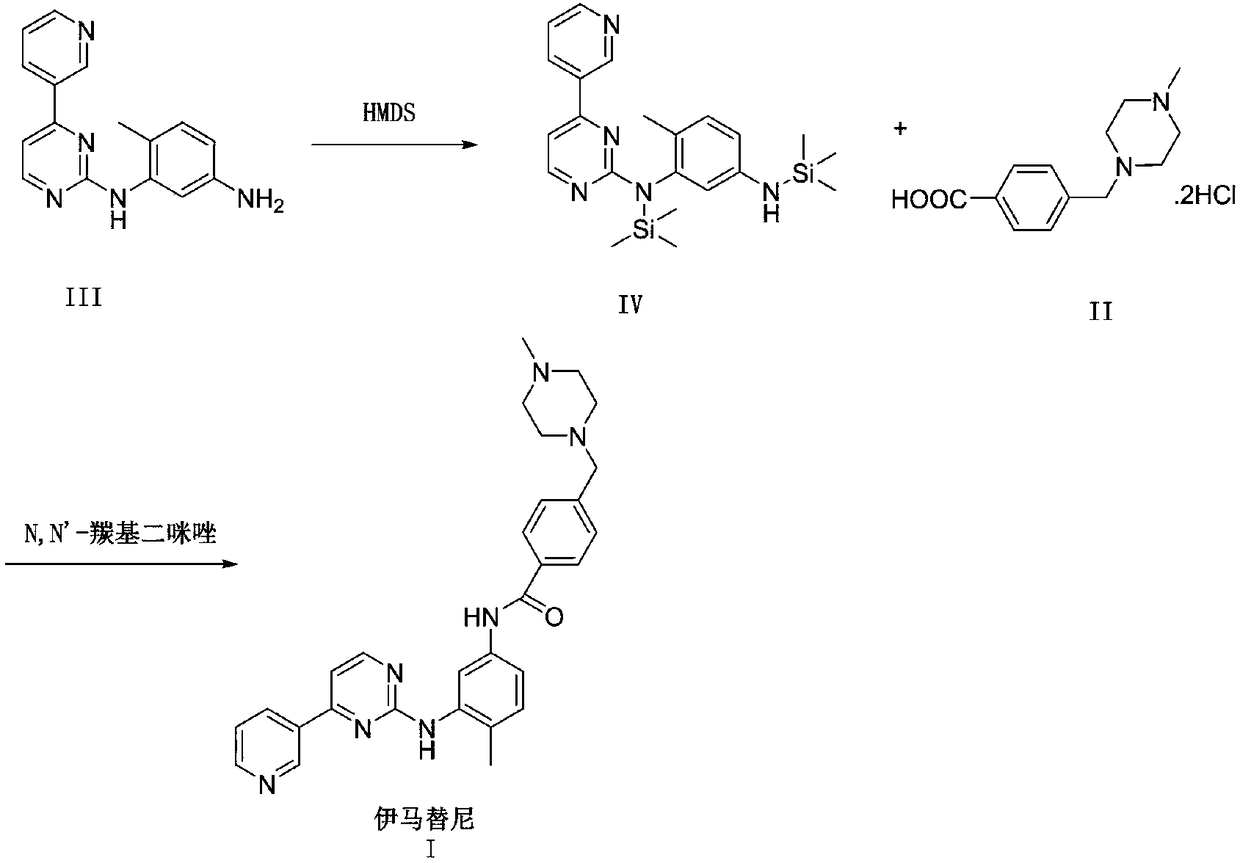
Synthesis method of imatinib
A synthesis method and technology of imatinib, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of large environmental pollution, large DMF-containing wastewater, large pollution, etc., and achieve large reagents, high reactivity, and product yield to avoid environmental pollution. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of compound (IV)
[0030] The first step, N-(5-amino-2-methylphenyl)-4-(3-pyridine)-2-pyrimidinamine 27.7g (100.0mmol, 1.0eq), hexamethyldisilazane 96.9g (600.0mmol, 6.0eq) was placed in a reaction flask, heated to reflux and reacted overnight, the reaction of the raw material in TLC was complete, the temperature was lowered, and the solvent and excess hexamethyldisilazane were concentrated under reduced pressure to obtain 41.3g of yellow oil , HPLC purity 95.3%, yield 98.1%. 1 H-NMR (400MHz, DMSO-d6): 0.11(s, 9H, NSi(CH3)3), 0.15(s, 9H, Si(CH3)3), 2.06(s, 3H, CH3), 2.84(broad, 1H,NH),6.33(dd,1H,J=8.0 and2.3Hz,Ph-H-4),6.71(d,1H,J=2.3Hz,Ph-H-2),6.79(d,1H,J =8.0Hz, Ph-H-5),7.22(d,1H,J=5.2Hz,pyrim-H-5),7.51(ddd,1H,J=8.0 and 4.8Hz,pyr-H-5),8.25 (td,1H,J=8.0 and 1.7Hz,pyr-H-4),8.42(d,1H,J=5.2Hz,pyrim-H-6),8.60(dd,1H,J=4.8 and1.7Hz, pyr-H-6), 9.17 (d, 1H, J = 1.7 Hz, pyr-H-2) ppm. ESI + [M + H] + = 422.2.
[0031] Synthesis of compound (I)
[0032] In the seco...
Embodiment 2
[0034] Synthesis of compound (IV)
[0035] The first step, N-(5-amino-2-methylphenyl)-4-(3-pyridine)-2-pyrimidinamine 138.5g (0.5mol, 1.0eq), solid ammonium chloride 2.8g, hexamethyl Hexamethyldisilazane 242.0g (1.5mol, 3.0eq) was placed in a reaction flask, heated to reflux for 5 hours, the reaction of the raw materials in TLC was complete, the temperature was lowered, and the solvent and excess hexamethyldisilazane were concentrated under reduced pressure to obtain 209.7 g of yellow oil, HPLC purity 95.7%, yield 99.6%. ESI+[M+H]+=422.2.
[0036] Synthesis of compound (I)
[0037]In the second step, 4-[(4-methyl-1-piperazine) methyl] benzoic acid dihydrochloride 168.3g (0.548mol, 1.1eq) and 500g methylene chloride are put in the reaction flask, and the temperature control is 10- Add 28.2g (218.2mmol, 2.2eq) of diisopropylethylamine at 20°C and stir for 30 minutes, then add 6.5g (49.8mmol, 1%eq) of tetrabutylammonium fluoride, and add N,N' in batches -Carbonyldiimidazole 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com