Anti-tumor compound with phototoxicity and preparation method and application thereof
An anti-tumor and phototoxic technology, applied in ruthenium organic compounds, platinum group organic compounds, anti-tumor drugs, etc., can solve the problems of complex and difficult clinical application of side effects, and achieve the effect of molecular stability, simple method, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of the phototoxic antitumor compound of the present invention is:
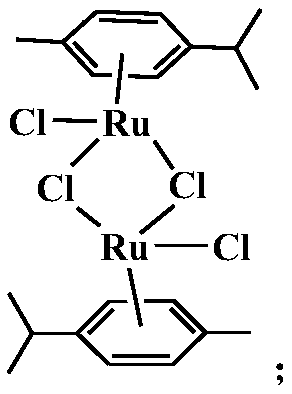
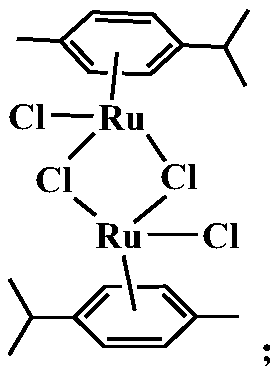
[0035] S1, the RuCl of 350mg ruthenium weight content 37% 3 ·xH 2 O and 2.8mL of γ-terpinene with a purity of 95% were mixed and dissolved in 9mL of absolute ethanol, and the temperature was refluxed at 40°C for 6h, and the intermediate product (dichlorodimethylcumene dichloride combined diruthenium), the chemical formula of the intermediate product is:
[0036]
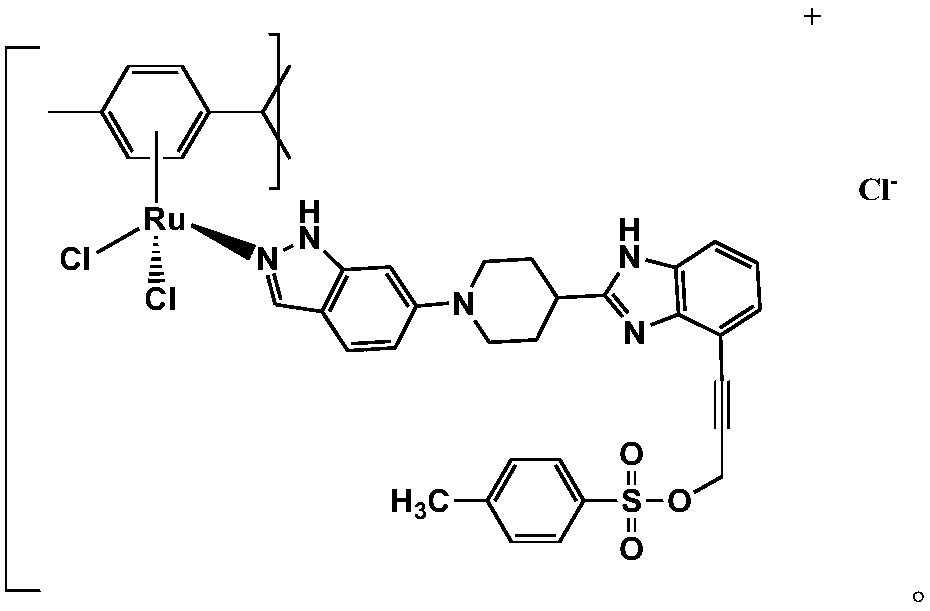
[0037] S2. Weigh 200 mg of 2-(4-piperidinyl) benzimidazole, then add 19 mL of carbon tetrachloride solvent, 0.8 mg of azobisisobutyronitrile, and 170 mg of N-bromosuccinyl Amine, reflux at 60°C for 20h, remove the solvent by rotary evaporation, then add 118mg of 6-bromoindazole and 31mg of the intermediate product, dissolve them in 14mL of anhydrous toluene, add 0.18mg of NaOH, 0.08mL of ethylenediamine, 2.8mg of cesium carbonate, 5.5mL of triethylamine, and then 30min of nitrogen, and then sequentially added 14.5m...
Embodiment 2
[0039] The preparation method of the phototoxic antitumor compound of the present invention is:
[0040] S1, the RuCl of 366mg ruthenium weight content 37% 3 ·xH 2 O and 3 mL of γ-terpinene with a purity of 95% are mixed and dissolved in 10 mL of absolute ethanol, and the temperature is refluxed for 6 h at 40° C., and the intermediate product (dichlorodimethylcumene dichloride) is separated out after standing. diruthenium), the chemical formula of the intermediate product is:
[0041]
[0042] S2. Weigh 201 mg of 2-(4-piperidinyl) benzimidazole, then add 20 mL of carbon tetrachloride solvent, 1 mg of azobisisobutyronitrile, and 177 mg of N-bromosuccinimide , reflux at 60°C for 24 hours, remove the solvent by rotary evaporation, then add 120 mg of 6-bromoindazole and 31.5 mg of the intermediate product, dissolve them in 15 mL of anhydrous toluene, add 0.2 mg of NaOH, 0.1mL of ethylenediamine, 3mg of cesium carbonate, 6mL of triethylamine, and then 30min of nitrogen, and t...
Embodiment 3
[0044] The preparation method of the phototoxic antitumor compound of the present invention is:
[0045] S1, the RuCl of 370mg ruthenium weight content 37% 3 ·xH 2O and 3.2mL of γ-terpinene with a purity of 95% were mixed and dissolved in 11mL of absolute ethanol, and the temperature was refluxed at 60°C for 8h, and the intermediate product (dichlorodimethylcumene dichloride combined diruthenium), the chemical formula of the intermediate product is:
[0046]
[0047] S2. Weigh 203 mg of 2-(4-piperidinyl) benzimidazole, then add 21 mL of carbon tetrachloride solvent, 1.2 mg of azobisisobutyronitrile, and 180 mg of N-bromosuccinyl Amine, reflux at 70°C for 26 hours, remove the solvent by rotary evaporation, then add 122 mg of 6-bromoindazole and 32 mg of the intermediate product, dissolve them in 15.5 mL of anhydrous toluene, and add 0.22 mg of NaOH under stirring conditions , 0.12mL of ethylenediamine, 3.2mg of cesium carbonate, 6.5mL of triethylamine, and then 30min of n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com