Preparation method of caprolactam and N-substituted caprolactam
A technology of caprolactam and caprolactone, which is applied in the field of preparation of caprolactam and N-substituted caprolactam, can solve the problems of a large amount of waste water, equipment corrosion, environmental pollution, etc., and achieves the effects of low cost, favorable industrial production, and simple catalytic process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
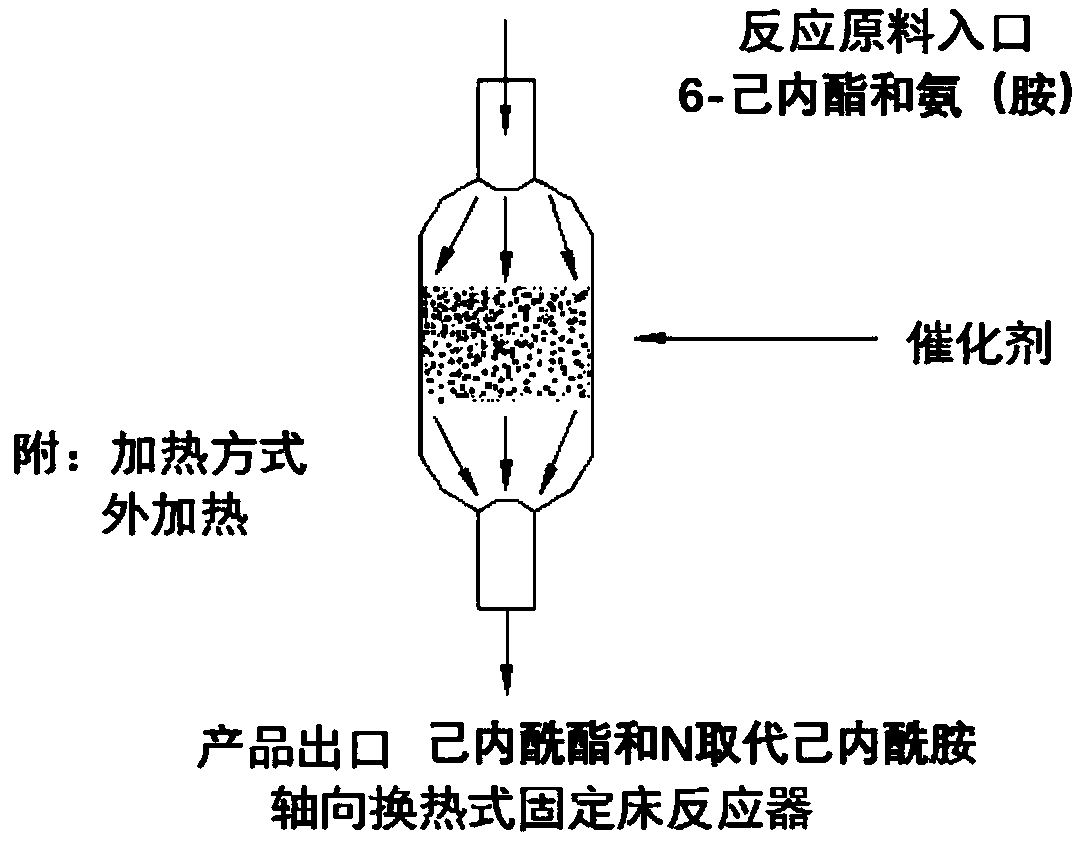
[0025] see figure 1 , the present invention provides the following technical solutions: a preparation method of caprolactam and N-substituted caprolactam, comprising the following steps:
[0026] (1) Add catalyst: put Φ=4.0~6.0mm granular SO in the fixed bed 4 2- / M x o y 150.0-200.0g quasi-solid superacid catalyst, SO 4 2- / M x o y The quasi-solid superacid refers to the solid superacid described in patent CN02151526.3, and the amine is the structure RNH 2 A series of compounds, including ammonia water, alkylamine (methylamine aqueous solution, ethylamine aqueous solution, propylamine, butylamine, octylamine, etc.), aromatic amines (aniline, substituted aniline, polycyclic amine, etc.), ethanolamine, etc.;
[0027] (2) Heating: heating to raise the temperature to 180.0-320.0°C, preferably 220.0-280.0°C;
[0028] (3) Introduce nitrogen: at a space velocity of 50.0 to 70.0cm 3 / min Feed nitrogen into the fixed bed, so that the catalyst is always in a state of nitrogen...
Embodiment 1
[0035] Embodiment one: (1) add catalyst: load the particle type SO of Φ=4.0~6.0mm in the fixed bed 4 2- / M x o y 200.0 g of quasi-solid superacid catalyst;
[0036] (2) Heating: heating to raise the temperature to 280.0°C;
[0037] (3) Introduce nitrogen: at a space velocity of 70.0cm 3 / min Feed nitrogen into the fixed bed, so that the catalyst is always in a state of nitrogen protection;
[0038] (4) Mixing of raw materials: 6-caprolactone and ammonia water are mixed in a molar ratio of 6-caprolactone:ammonia=1.1:1.0 at room temperature;
[0039] (5) Input the mixed solution of 6-caprolactone and ammoniacal liquor, import the mixed solution of 6-caprolactone and ammoniacal liquor to fixed bed with weight space velocity 0.8g / min, add 6-caprolactone and ammonia gross weight 1.0 simultaneously twice as much water as a solvent;
[0040] (6) Product generation: 6-caprolactone and ammonia water are dehydrated under the continuous catalytic action of a hot catalyst to genera...
Embodiment 2
[0043] Embodiment two: (1) add catalyzer: load the particle type SO of Φ=4.0~6.0mm in fixed bed 42- / M x o y 180.0 g of quasi-solid superacid catalyst;
[0044] (2) Heating: heating to raise the temperature to 240.0°C;
[0045] (3) Introduce nitrogen: at a space velocity of 50cm 3 / min Feed nitrogen into the fixed bed, so that the catalyst is always in a state of nitrogen protection;
[0046] (4) Mixing of raw materials: Mix 6-caprolactone and aniline at room temperature with a molar ratio of 6-caprolactone:amine=1.2:1.0, and add benzene which is 2.0 times the total weight of 6-caprolactone and amine as a solvent ;
[0047] (5) input the mixed solution of 6-caprolactone and amine, input the mixed solution of 6-caprolactone and amine to fixed bed with weight space velocity 1.0g / min;
[0048] (6) Product generation: 6-caprolactone and amine are dehydrated under the continuous catalytic action of a hot catalyst to generate N-phenylcaprolactam;
[0049] (7) Product post-tre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com