Reduced responsive drug-drug conjugate preparation method
A conjugate and responsive technology, which is applied in the field of preparation of reduction-responsive drug-drug conjugates, can solve the problems of large drug side effects, high carrier mass ratio, and low drug loading, and achieve the effect of increasing the loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
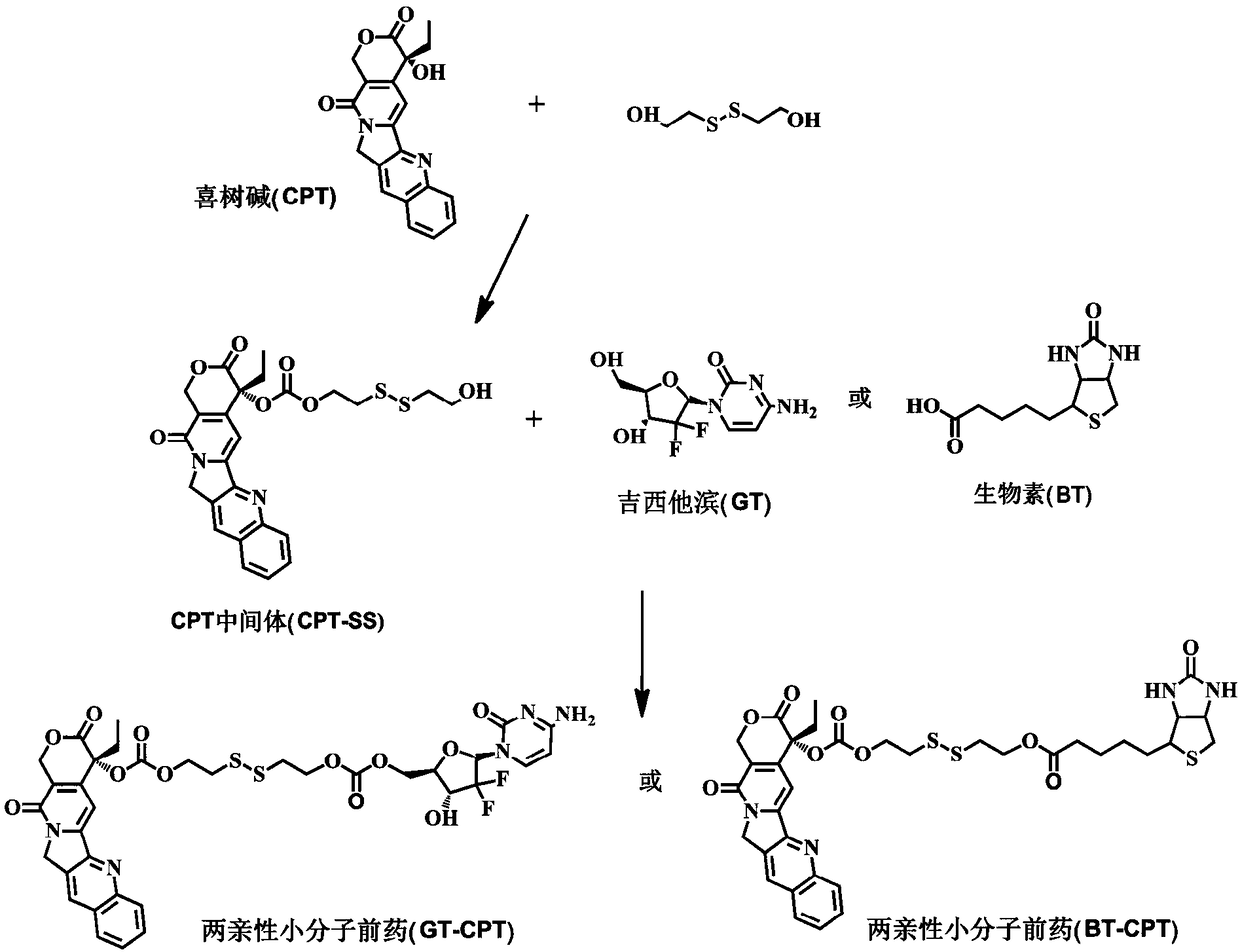
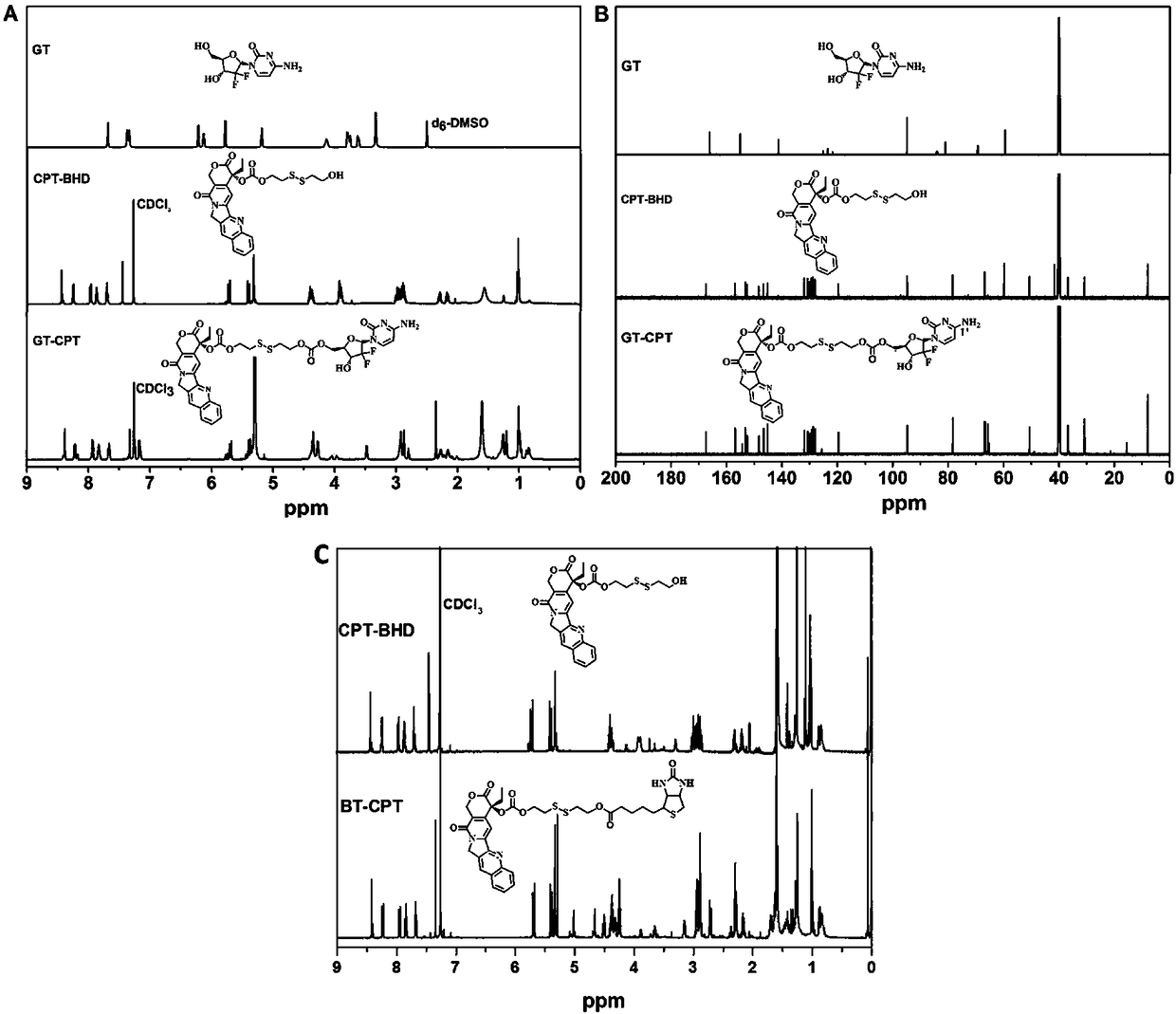
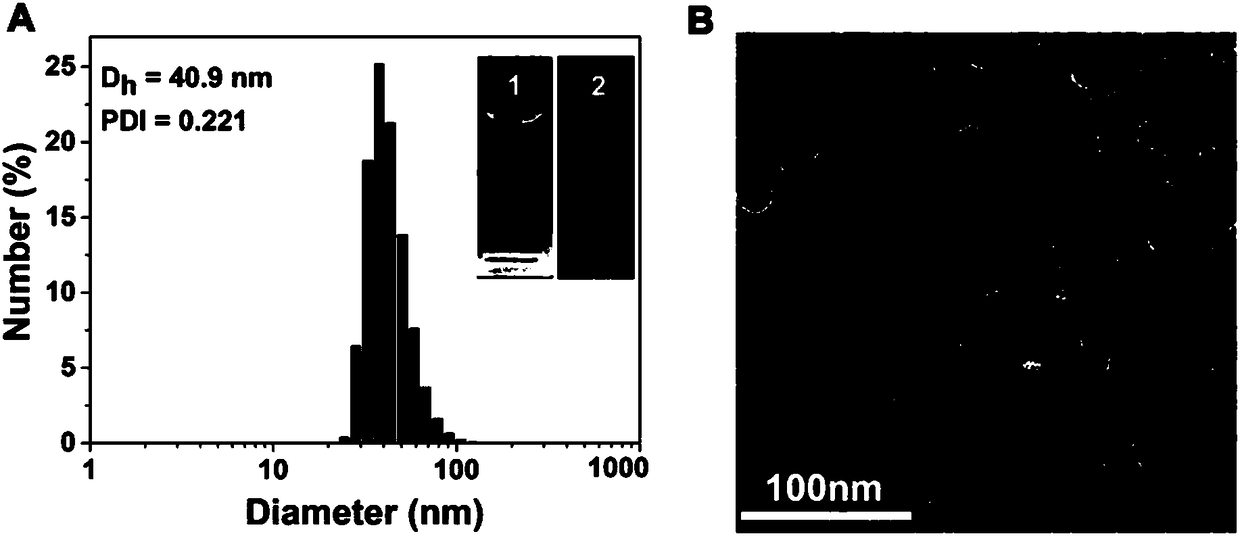
[0023] Such as Figure 1 to Figure 7 Shown, a kind of preparation method of reduction-responsive drug-drug conjugate comprises the following steps:
[0024] The first step is to prepare the camptothecin intermediate (CPT-SS) containing reduction-responsive bonds: under the condition of argon (Ar) reaching 5-10Pa, the original molecule of camptothecin (CPT) is first dissolved in Water dichloromethane (DCM) solvent, then add 4-dimethylaminopyridine (DMAP) and solid triphosgene respectively, the molar ratio of the three drugs is 1:3:1 / 3, after stirring for 20 minutes under dark conditions , then slowly drop into anhydrous tetrahydrofuran (THF) solution of dithiodiethanol (BHD) 5 times the molar amount of CPT, and stir overnight; then, after extraction and column chromatography purification, pure monohydroxyl substituted camptothecin intermediate (CPT-SS);
[0025] In the second step, preparation of reduction-responsive drug-drug conjugates:
[0026] Under the condition of temp...
Embodiment 2
[0034] The first step in the preparation steps is completely consistent with the specific embodiment one, only the second step of the preparation steps is different from the specific embodiment one, and its specific preparation process is as follows:
[0035] The specific steps for preparing the reduction-responsive drug-drug conjugate BT-CPT are as follows: at a temperature ≤ 0°C and in an atmosphere where argon (Ar) reaches 5-10Pa, the camptophyllin prepared in the first step Base intermediate (CPT-SS), biotin (BT) dissolved in anhydrous N,N-dimethylformamide (DMF) solution, the above camptothecin intermediate (CPT-SS), biotin (BT ) The molar ratio of the two drugs is 1:1. After stirring for 30 minutes at 0 ℃ and protected from light, add 1-ethyl-(3-dimethylaminopropyl) carbonyl dicarboxylate with 2 times the molar amount of biotin Imine hydrochloride (EDC·HCl) and 0.1 times biotin molar amount of 4-dimethylaminopyridine (DMAP) in N,N-dimethylformamide (DMF) solution, raised...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com