Preparation method for dehydrogenation catalyst
A dehydrogenation catalyst and catalyst technology, applied in the direction of chemical instruments and methods, heterogeneous catalyst chemical elements, physical/chemical process catalysts, etc., can solve the problems of insignificant effect, poor recycling performance, etc., achieve high activity, reduce Operating cost, the effect of prolonging the one-way operation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
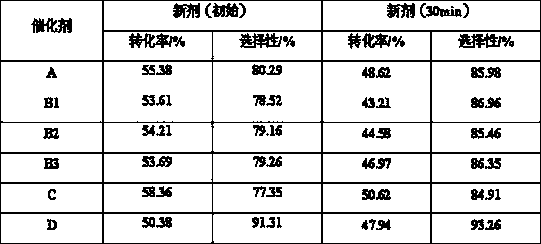
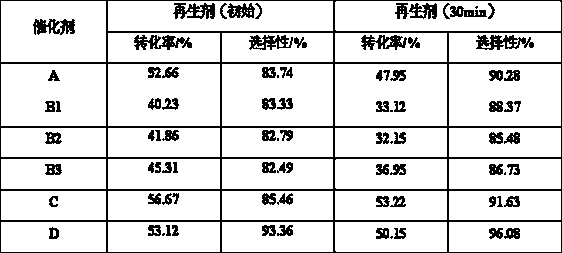
Image
Examples
Embodiment 1
[0027] Take 200 mL each of deionized water and absolute ethanol, and mix well at room temperature. Weigh 45g of benzoic acid and dissolve in the above solvent, stir well and heat the solvent to 60°C in a water bath. Then add an appropriate amount of chromium oxalate to the above solution, stir until it is completely dissolved, then add an appropriate amount of lanthanum chloride, adjust the pH of the solution to 5 with dilute hydrochloric acid, and stir until it is completely dissolved.
[0028] Weigh 300mL γ-Al 2 o 3 The carrier is placed in a rotary evaporator, vacuumed for 30 minutes, then the above solution is impregnated and pumped into a rotary bottle, and the carrier is impregnated for 8 hours under normal pressure. Evaporate the impregnation solution at 80°C to dryness, unload the catalyst, put it in an oven to dry at 120°C for 5 hours, and put it into a muffle furnace for calcination at 550°C for 4 hours.
[0029] The above-mentioned catalyst is then impregnated wi...
Embodiment 2
[0044] Take 200mL of deionized water and 240mL of absolute ethanol, and mix well at room temperature. Weigh 55g of benzoic acid and dissolve it in the above solvent, stir well and heat the solvent to 70°C in a water bath. Then add an appropriate amount of chromium oxalate to the above solution, stir until it is completely dissolved, then add an appropriate amount of lanthanum chloride, adjust the pH of the solution to 5.5 with dilute hydrochloric acid, and stir until it is completely dissolved.
[0045] Weigh 280mL γ-Al 2 o 3 The carrier is placed in a rotary evaporator, vacuumed for 30 minutes, then the above solution is impregnated and pumped into a rotary bottle, and the carrier is impregnated for 6 hours under normal pressure. Evaporate the impregnating solution at 75°C to dryness, unload the catalyst, put it in an oven to dry at 110°C for 4 hours, and put it into a muffle furnace for calcination at 600°C for 4 hours.
[0046] The above catalyst is impregnated with an a...
Embodiment 3
[0048] Take 250mL of deionized water and 190mL of absolute ethanol, and mix well at room temperature. Weigh 39g of benzoic acid and dissolve in the above solvent, stir well and heat the solvent to 75°C in a water bath. Then add an appropriate amount of chromium oxalate to the above solution, stir until it is completely dissolved, then add an appropriate amount of lanthanum chloride, adjust the pH of the solution to 4.6 with dilute hydrochloric acid, and stir until it is completely dissolved.
[0049] Weigh 260mL γ-Al 2 o 3 The carrier was placed in a rotary evaporator, vacuumed for 30 minutes, then the above solution was impregnated and pumped into a rotary bottle, and the carrier was impregnated for 5 hours under normal pressure. Evaporate the impregnating solution at 80°C, unload the catalyst, dry in an oven at 110°C for 6 hours, and then put it in a muffle furnace for 3 hours at 560°C.
[0050] The above-mentioned catalyst is then impregnated with an aqueous solution of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com