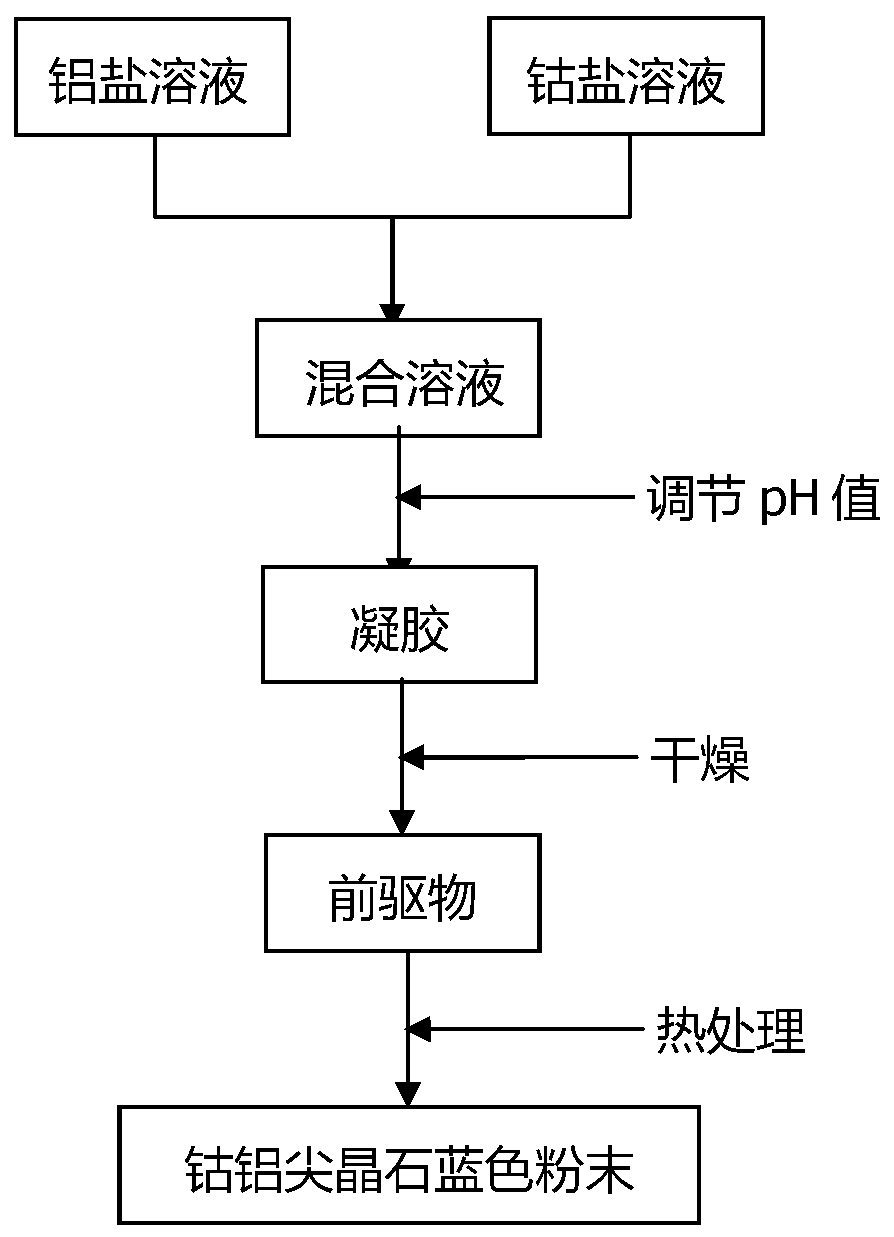
Method for preparing blue cobalt aluminate by sol gel method
A sol-gel method, aluminum spinel technology, applied in chemical instruments and methods, cobalt compounds, nanotechnology for materials and surface science, etc., can solve the reduction of powder specific surface area, stoichiometric ratio deviation, application Due to the limited field and other problems, the effects of high material strength and toughness, positive product color, and wide doping range are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
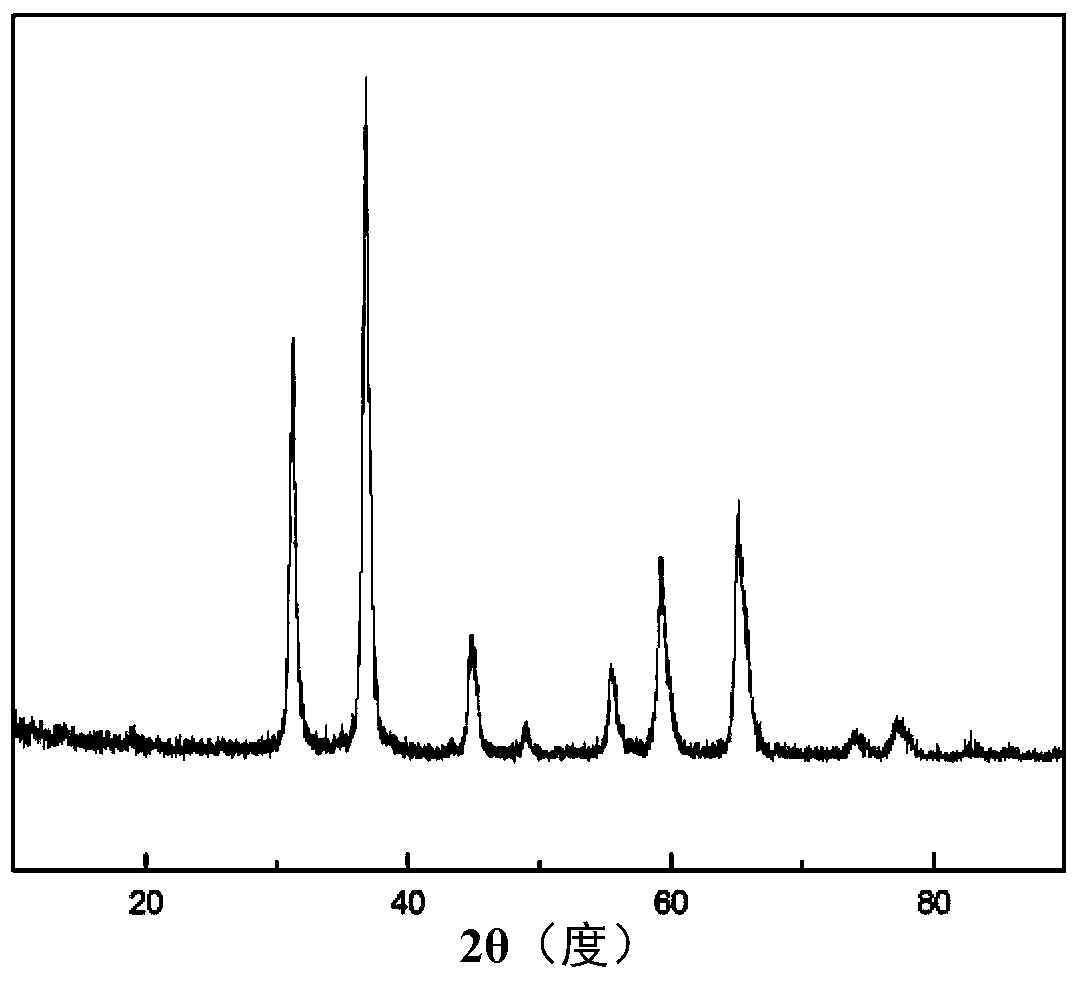
[0046] Using aluminum chloride and cobalt chloride as raw materials, according to Co 2+ :Al 3+ Molar ratio=1:4 batching, specific operation steps are as follows: respectively dissolve aluminum chloride, cobalt chloride in deionized water, make solution, wherein the concentration of aluminum chloride solution is 0.2mol / L, the concentration of cobalt chloride solution The concentration is 0.2mol / L. After mixing, a sol is formed and kept stirring. Adjust the pH to 5 with oxalic acid solution. After stirring fully, let the solution stand for 12 hours to precipitate, centrifuge (suction filtration), wash and dry. The above powder was heat-treated in a muffle furnace at 1000°C for 2 hours to obtain the final sample. Its XRD pattern is as figure 2 ,From figure 2 Clear CoAl can be seen in 2 o 4 The diffraction peaks correspond to (220), (311), (400), (422), (511) and (440) crystal planes in (PDF 44-0160), which proves that CoAl 2 o 4 Powder. . Its TEM pictures are as Figu...
Embodiment 2
[0048] Using aluminum chloride and cobalt chloride as raw materials, according to Co 2+ :Al 3+ Molar ratio=1:2 batching, specific operation steps are as follows: respectively dissolve aluminum chloride, cobalt chloride in deionized water, make solution, wherein the concentration of aluminum chloride solution is 0.3mol / L, the concentration of cobalt chloride solution The concentration is 0.3mol / L. After mixing, a sol is formed and kept stirring. Adjust the pH to 4 with oxalic acid solution. After stirring fully, let the solution stand for 24 hours to precipitate, centrifuge (suction filtration), wash and dry. The above powder was heat-treated in a muffle furnace at 1200°C for 3 hours to obtain the final sample. Its XRD pattern is as image 3 ,From image 3 Clear CoAl can be seen in 2 o 4 The diffraction peaks correspond to (220), (311), (400), (422), (511) and (440) crystal planes in (PDF 44-0160), respectively, and the peaks are strong and sharp, indicating that the obtai...
Embodiment 3
[0050] Using aluminum sulfate and cobalt sulfate as raw materials, according to Co 2+ :Al 3+ Molar ratio=1:2 ingredients, the specific operation steps are as follows: respectively dissolve aluminum sulfate and cobalt sulfate in deionized water to make a solution, wherein the concentration of aluminum sulfate solution is 0.2mol / L, and the concentration of cobalt sulfate solution is 0.2mol / L, form a sol after mixing and keep stirring, adjust the pH to 6 with oxalic acid solution, after stirring fully, let the solution stand for 18 hours to precipitate, centrifuge (suction filtration) wash, and dry. The above powder was heat-treated in a muffle furnace at 800 °C for 5 h to obtain the final sample. Its XRD pattern is as Figure 4 ,From Figure 4 Clear CoAl can be seen in 2 o 4 The diffraction peaks correspond to (220), (311), (400), (422), (511) and (440) crystal planes in (PDF 44-0160), respectively, and the peaks are strong and sharp, indicating that the obtained CoAl 2 o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap